Abstract
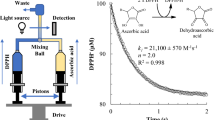
This work determines the radical scavenging activity of antioxidants and berry extracts based on the heat generated during their reaction with hydrogen peroxide, under isothermal condition (25 °C). After addition of H2O2 to a water solution containing antioxidants, an exothermic heat flow appeared. After an initial damping time, the signal decayed exponentially, following a first-order kinetic. Through an iterative fitting routine, both thermodynamic (ΔH) and kinetic (k) information were achieved. Such approach was applied toward relevant food antioxidants, revealing that the fastest reactivity (k) was for tannic acid > gallic acid > caffeic acid > ascorbic acid. Interestingly, k was inversely correlated with ΔH (r = −0.96) and with the DPPH test (r = −0.98). Apparently, strong radical scavengers show faster kinetics and lower ΔH-values, as expected, respectively, from a high reactivity toward peroxyl radical and efficient delocalization capacity. Such approach was finally applied to berry extracts (mixed grape seed and skin; chokeberries; grape seed; goji berries). The resulting ΔH-values were correlated with three indices, namely, total phenol, amperometry, and DPPH test. However, k-values largely deviated from these indices. Such discrepancy was explained considering that none of these indices is a “true” measure of the kinetic of the reaction, but only express an apparent concentration. Conversely, reaction calorimetry provides directly and simultaneously both thermodynamic and kinetic properties of the radical scavenging reactivity of antioxidants or natural extracts.





Similar content being viewed by others
References
Ak T, Gülçin İ (2008) Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact 174(1):27–37
Bartosz G (2006) Use of spectroscopic probes for detection of reactive oxygen species. Clin Chim Acta 368(1):53–76
Bozin B, Mimica-Dukic N, Samojlik I, Goran A, Igic R (2008) Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae). Food Chem 111(4):925–929
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28(1):25–30
Forino M, Tartaglione L, Dell’Aversano C, Ciminiello P (2016) NMR-based identification of the phenolic profile of fruits of Lycium barbarum (goji berries). Isolation and structural determination of a novel N-feruloyl tyramine dimer as the most abundant antioxidant polyphenol of goji berries. Food Chem 194:1254–1259
Foti MC (2015) Use and abuse of the DPPH• radical. J Agric Food Chem 63(40):8765–8776
Gaisford S, Hills AK, Beezer AE, Mitchell JC (1999) Thermodynamic and kinetic analysis of isothermal microcalorimetric data: applications to consecutive reaction schemes. Thermochim Acta 328(1):39–45
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930
Gomes A, Fernandes E, Lima JLFC (2005) Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Methods 65(2):45–80
Gorinstein S, Arancibia-Avila P, Toledo F, Namiesnik J, Leontowicz H, Leontowicz M, Kyung-Sik H, Seong-Gook K, Vearasilp K, Suhaj M (2013) Application of analytical methods for the determination of bioactive compounds in some berries. Food Anal Methods 6(2):432–444
Kamrul HSM, Schiraldi A, Cosio MS, Scampicchio M (2016) Food and ascorbic scavengers of hydrogen peroxide. J Therm Anal Calorim 125(2):729–737
Liang N, Kitts DD (2014) Antioxidant property of coffee components: assessment of methods that define mechanisms of action. Molecules 19(11):19180–19208
McDougall GJ, Austin C, Van Schayk E, Martin P (2016) Salal (Gaultheria shallon) and aronia (Aronia melanocarpa) fruits from Orkney: phenolic content, composition and effect of wine-making. Food Chem 205:239–247
Nilsson H, Hess U (2008) Introduction of a calibration-free reaction calorimeter that combines the benefits of DSCS and reaction calorimeters. J Therm Anal Calorim 93(1):219–224
Özyürek M, Bektaşoğlu B, Güçlü K, Güngör N, Apak R (2010) A novel hydrogen peroxide scavenging assay of phenolics and flavonoids using cupric reducing antioxidant capacity (CUPRAC) methodology. J Food Compos Anal 23(7):689–698
Pick E, Keisari Y (1981) Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages—induction by multiple nonphagocytic stimuli. Cell Immunol 59(2):301–318
Ruch RJ, Cheng S, Klaunig JE (1989) Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 10(6):1003–1008
Sroka Z, Cisowski W (2003) Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem Toxicol 41(6):753–758
Wang SY, Jiao H (2000) Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. J Agric Food Chem 48(11):5677–5684
Willson RJ, Beezer AE, Mitchell JC, Loh W (1995) Determination of thermodynamic and kinetic parameters from isothermal heat conduction microcalorimetry: applications to long-term-reaction studies. J Phys Chem 99(18):7108–7113
Yahui L, Xiaobo Z, Tingting S, Jiyong S, Jiewen Z, Holmes M (2017) Determination of geographical origin and anthocyanin content of black goji berry (Lycium ruthenicum Murr.) using near-infrared spectroscopy and Chemometrics. Food Anal Methods 10(4):1034–1044
Yen GC, Chen HY (1995) Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem 43(1):27–32
Zhou L, Elias RJ (2011) Investigating the hydrogen peroxide quenching capacity of proteins in polyphenol-rich foods. J Agric Food Chem 59(16):8915–8922
Acknowledgements
We are grateful to the Province of Bolzano for financial support (Landesregierung mittels Beschluss Nr. 1472, 07.10.2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Kamrul Hasan, S.M., Asaduzzaman, M., Merkyte, V. et al. Simultaneous Kinetic and Thermodynamic-Based Assay to Determine the Hydrogen Peroxide (H2O2) Scavenging Activity of Berry Extracts by Using Reaction Calorimetry. Food Anal. Methods 11, 432–439 (2018). https://doi.org/10.1007/s12161-017-1014-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-1014-z