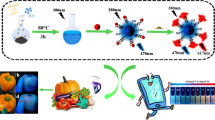
Abstract
Poly(5-sulfosalicylic acid) (PSSA)/Cu(OH)2 nanoparticle–graphite (Gr) nanocomposite-modified glassy carbon electrode (PSSA/Cu(OH)2–Gr/GCE) was utilized for sensitive determination of tartrazine using squarewave voltammetry (SWV). The structure of the nanocomposite was investigated by transmission electron microscopy (TEM) and field emission scanning electron microscopy (FESEM(. PSSA/Cu(OH)2–Gr/GCE exhibited an enhancement in anodic peak current, electron transfer kinetics, effective surface area, and reactive sites and indicated good electrocatalytic activity toward the oxidation of tartrazine. The as-proposed modified electrode achieved a satisfactory dynamic range between the anodic peak current and the concentration of tartrazine at two concentration ranges of 0.01–0.6 and 0.6–10 μmol/L, and the detection limit was obtained to be 8 nmol/L (S/N = 3). The resulting sensor was successfully used to determine tartrazine in real samples such as candy, softdrink, orange juice powder, banana-flavored jelly powder, and candy-coated chocolate.








Similar content being viewed by others
References
Alves SP, Brum DM, De Andrade ECB, Netto ADP (2008) Determination of synthetic dyes in foodstuff by high performance liquid chromatography with UV–DAD detection. Food Chem 107:489–496
Arvand M, Gholizadeh TM, Zanjanchi MA (2012) MWCNTs/Cu(OH)2 nanoparticles/IL nanocomposite modified glassy carbon electrode as a voltammetric sensor for determination of the non–steroidal anti–inflammatory drug diclofenac. Mater Sci Eng C 32:1682–1689
Ba X, Luo L, Ding Y, Liu X (2013) Determination of l–tryptophan in the presence of ascorbic acid and dopamine using poly(sulfosalicylic acid) modified glassy carbon electrode. Sensors Actuators B Chem 187:27–32
Bard AJ, Faulkner LR (2004) Electrochemical methods: fundamentals and applications. 2nd ed. Wiley
De León-Rodríguez LM, Basuil-Tobias DA (2005) Testing the possibility of using UV–Vis spectrophotometric techniques to determine non–absorbing analytes by inclusion complex competition in cyclodextrins. Anal Chim Acta 543:282–290
Devamani RHP, Alagar M (2013) Synthesis and characterisation of copper(II) hydroxide nano Particles. Nano Biomed Eng 5:116–120
Diacu E, Ungureanu EM, Ene CP, Ivanov AA (2011) Voltammetric studies for detection and degradation assessment of some synthetic food dyestuffs. I. Tartrazine–E 102. Rev Chim 62:1085–1089
Feng LJ, Zhang XH, Liu P, Xiong HY, Wang SF (2011) An electrochemical sensor based on single–stranded DNA–poly(sulfosalicylic acid) composite film for simultaneous determination of adenine, guanine, and thymine. Anal Biochem 419:71–75
Gan T, Sun J, Cao S, Gao F, Zhang Y, Yang Y (2012) One–step electrochemical approach for the preparation of graphene wrapped–phosphotungstic acid hybrid and its application for simultaneous determination of sunset yellow and tartrazine. Electrochim Acta 74:151–157
Gan T, Sun J, Meng W, Song L, Zhang Y (2013) Electrochemical sensor based on graphene and mesoporous TiO2 for the simultaneous determination of trace colourants in food. Food Chem 141:3731–3737
Gholivand MB, Torkashvand M (2016) The fabrication of a new electrochemical sensor based on electropolymerization of nanocomposite gold nanoparticle–molecularly imprinted polymer for determination of valganciclovir. Mater Sci Eng C 59:594–603
Ghoreishi SM, Behpour M, Golestaneh M (2012) Simultaneous determination of sunset yellow and tartrazine in soft drinks using gold nanoparticles carbon paste electrode. Food Chem 132:637–641
Gómez M, Arancibia V, Rojas C, Nagles E (2012) Adsorptive stripping voltammetric determination of tartrazine and sunset yellow in gelatins and soft drink powder in the presence of cetylpyridinium bromide. Int J Electrochem Sci 7:7493–7502
Gosser DK (1993) Cyclic voltammetry: simulation and analysis of reaction mechanisms. Wiley, New York
Laviron E (1974) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem 101:19–28
Ma M, Luo X, Chen B, Su S, Yao S (2006) Simultaneous determination of water–soluble and fat-soluble synthetic colorants in foodstuff by high–performance liquid chromatography-diode array detection–electrospray mass spectrometry. J Chromatogr A 1103:170–176
Majidi MR, Baj RFB, Naseri A (2013) Carbon nanotube–ionic liquid (CNT–IL) nanocomposite modified sol–gel derived carbon–ceramic electrode for simultaneous determination of sunset yellow and tartrazine in food samples. Food Anal Methods 6:1388–1397
Minioti KS, Akellariou CFS, Thomaidis NS (2007) Determination of 13 synthetic food colorants in water–soluble foods by reversed–phase high–performance liquid chromatography coupled with diode–array detector. Anal Chim Acta 583:103–110
Mokhtari A, Karimi–Maleh H, Ensafi AA, Beitollahi H (2012) Application of modified multiwall carbon nanotubes paste electrode for simultaneous voltammetric determination of morphine and diclofenac in biological and pharmaceutical samples. Sensors Actuators B Chem 169:96–105
Moretto LM, Kalcher K (eds) (2015) Environmental analysis by electrochemical sensors and biosensors. Nanostructure Science and Technology. Springer, New York
Qiu X, Lu L, Leng J, Yu Y, Wang W, Jiang M, Bai L (2016) An enhanced electrochemical platform based on graphene oxide and multi–walled carbon nanotubes nanocomposite for sensitive determination of sunset yellow and tartrazine. Food Chem 190:889–895
Riyanto NV, Othman MR (2015) Electrosynthesis and characterization of Cu(OH)2/nanoparticle using Cu and Cu–PVC electrodes in alkaline solution. Int J Electrochem Sci 10:4911–4921
Ryvolova M, Preisler J, Foret F, Hauser P, Krasensky P, Paull B, Macka M (2009) Combined contactless conductometric, photometric, and fluorimetric single point detector for capillary separation methods. Anal Chem 82:129–135
Sahraei R, Farmany A, Mortazavi SS (2013) A nanosilver–based spectrophotometry method for sensitive determination of tartrazine in food samples. Food Chem 138:1239–1242
Shawish HMA, Ghalwa NA, Saadeh SM, Harazeen HE (2013) Development of novel potentiometric sensors for determination of tartrazine dye concentration in foodstuff products. Food Chem 138:126–132
Silva MLS, Garcia MBQ, Lima JLFC, Barrado E (2007) Voltammetric determination of food colorants using a poly(allylamine) modified tubular electrode in a multicommutated flow system. Talanta 72:282–288
Sorouraddin MH, Rostami A, Saadati M (2011) A simple and portable multi–colour light emitting diode based photocolourimeter for the analysis of mixtures of five common food dyes. Food Chem 127:308–313
Wang M, Zhao J (2015) Facile synthesis of Au supported on ionic liquid functionalized reduced graphene oxide for simultaneous determination of sunset yellow and tartrazine in drinks. Sensors Actuators B Chem 216:578–585
Wang C, Zou X, Zhao X, Wang Q, Tan J, Yuan R (2015) Cu–nanoparticles incorporated overoxidized–poly(3–amino–5-mercapto–1,2,4–triazole) film modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid and tryptophan. J Electroanal Chem 741:36–41
Xiao–Yan G, Jian–Cheng D (2007) Preparation and electrochemical performance of nano–scale nickel hydroxide with different shapes. Mater Lett 61:621–625
Ye X, Du Y, Lu D, Wang C (2013) Fabrication of ?–cyclodextrin–coated poly (diallyldimethylammonium chloride)–functionalized graphene composite film modified glassy carbon–rotating disk electrode and its application for simultaneous electrochemical determination colorants of sunset yellow and tartrazine. Anal Chim Acta 779:22–34
Zhang W, Liu T, Zheng X, Huang W, Wan C (2009) Surface–enhanced oxidation and detection of sunset yellow and Tartrazine using multi–walled carbon nanotubes film–modified electrode. Colloids Surf B: Biointerfaces 74:28–31
Zhao L, Zeng B, Zhao F (2014) Electrochemical determination of tartrazine using a molecularly imprinted polymer–multiwalled carbon nanotubes–ionic liquid supported Pt nanoparticles composite film coated electrode. Electrochim Acta 146:611–617
Zhao H, Zhang Y, Yuan Z (2001) Study on the electrochemical behavior of dopamine with poly(sulfosalicylic acid) modified glassy carbon electrode. Anal Chim Acta 441:117–122
Acknowledgements
The authors are thankful to the post-graduate office of Guilan University for the support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Guilan University.
Conflict of Interest
M. Arvand declares that he has no conflict of interest. A. Ashoori declares that she has no conflict of interest. Sh. Hemmati declares that she has no conflict of interest.
Ethical Approval
All procedures performed in studies were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with human or animal subjects performed by any of the authors.
Informed Consent
Not applicable.
Electronic supplementary material
ESM 1
(DOCX 405 kb.)
Rights and permissions
About this article
Cite this article
Arvand, M., Gaskarmahalleh, A.A. & Hemmati, S. Enhanced-Oxidation and Highly Sensitive Detection of Tartrazine in Foodstuffs via New Platform Based on Poly(5-Sulfosalicylic Acid)/Cu(OH)2 Nanoparticles. Food Anal. Methods 10, 2241–2251 (2017). https://doi.org/10.1007/s12161-016-0782-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0782-1