Abstract
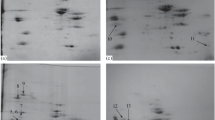
The aim of this study was to develop a simple method for simultaneous identification of two different domains of calpains (μ- and m-calpains) in blood and turkey meat samples. The method is based on Tris-based extraction techniques followed by casein zymography detection. The extracts were purified by dialysis, and target compounds were separated on Diethylaminoethyl (DEAE)-Sephacel anion-exchange chromatography. Results indicated that a buffer pH of 6.7 produced optimum extraction efficiency. The separation of calpastatin, μ- and m-calpains on column chromatography was carried out using stepwise elution profiles of 100, 200, and 400 mM NaCl solutions, respectively. Casein zymography study clearly demonstrated the presence of μ- and m-calpains in the crude extracts of breast and blood samples; however, μ-calpain was absent in the thigh sample. Similarly, sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis identified both the calpains from muscle and blood samples, but presence of calpastatin was observed in both the breast and thigh muscle samples only. The activity analysis of purified fractions indicated that both the μ- and m-calpain concentrations were differed significantly (P < 0.05) amongst breast, thigh and blood samples. Collectively, it was concluded that Tris buffer (pH 6.7) and DEAE-Sephacel column for extraction and separation of μ- and m-calpains and casein zymography and SDS-PAGE detection methods were most suitable for accurate identification of calpains and calpastatin from blood and turkey meat samples.


Similar content being viewed by others
References
Busch WA, Stromer MH, Goll DE, Suzuki A (1972) Ca2+−specific removal of Z-lines from rabbit skeletal muscle. J Cell Biol 52:367–381
Dayton WR, Goll DE, Zeece MG, Robson RM, Reville WJ (1976) A Ca2+ activated protease possibly involved in myofibrillar protein turnover. Biochem 15:2150–2158
Destefanis G, Brugiapaglia A, Barge MT, Dal Molin E (2008) Relationship between beef consumer tenderness perception and Warner-Bratzler shear force. Meat Sci 78:153–156
Etherington DJ, Taylor MAJ, Dransfield E (1987) Conditioning of meat from different species. Relationship between tenderising and the levels of cathepsin B, cathepsin L, calpain I, calpain II and β-glucuronidase. Meat Sci 20(1):1–18
Geesink GH, Koohmaraie M (1999) Effect of calpastatin on degradation of myofibrillar proteins by μ-calpain under post-mortem conditions. J Anim Sci 77:2685–2692
Geesink GH, Nonneman D, Koohmaraie M (1998) An improved purification protocol for heart and skeletal muscle calpastatin reveals two isoforms resulting from alternative splicing. Arch Biochem Biophys 356:19–24
Huang M, Huang F, Ma H, Xu X, Zhou G (2012) Preliminary study on the effect of caspase-6 and calpain inhibitors on post-mortem proteolysis of myofibrillar proteins in chicken breast muscle. Meat Sci 90:536–542
Koohmaraie M (1990) Quantification of Ca2+ dependent protease activities by hydrophobic and ion-exchange chromatography. J Anim Sci 68:659–665
Koohmaraie M (1994) Muscle proteinases and meat ageing. Meat Sci 36:93–104
Koohmaraie M, Geesink GH (2006) Contribution of post-mortem muscle biochemistry to the delivery of consistent meat quality with particular focus on the calpain system. Meat Sci 74:34–43
Kretchmar DH, Hathaway MR, Epley RJ, Dayton WR (1990) Alterations in postmortem degradation of myofibrillar proteins in muscle of lambs fed a beta-adrenergic agonist. J Anim Sci 68:1760–1772
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 277:680–685
Lee HS, Santé-Lhoutellier V, Vigouroux S, Briand Y, Briand M (2007) Calpain specificity and expression in chicken tissues. Comp Biochem Physiol 146:88–93
Maddock KR, Huff-Lonergan J, Lonergan E, Steven M (2005) The effect of pH on μ-calpain activity and implications in meat tenderness. Anim Indus Report. AS 651, ASL R1988.
Miller MF, Carr MF, Ramsey CB, Crockett KL, Hoover LC (2001) Consumer thresholds for establishing the value of beef tenderness. J Anim Sci 79:3062–3068
Obanor F, Morton JD, Geesink GH, Bickerstaffe R (2005) Effect of processing on turkey meat quality and proteolysis. Poult Sci 84:1123–1128
Salem M, Kenney PB, Killefer J, Nath J (2004) Isolation and characterization of calpains from rainbow trout muscle and their role in texture development. J Muscle Foods 15(4):245–255
Snedecor GW, Cochran WG (1994) Statistical methods, 8th edn. New Delhi. Oxford and IBH Pub, Co
Taylor RG, Geesink GH, Thompson VF, Koohmaraie M, Goll DE (1995) Is Z-disk degradation responsible for post-mortem tenderization? J Anim Sci 73:1351–1367
Veiseth E, Shackelford SD, Wheeler TL, Koohmaraie M (2001) Effect of post-mortem storage on μ-calpain and m-calpain in ovine skeletal muscle. J Animal Sci 79:1502–1508
Vidalenc P, Cottin P, Merdaci N, Ducastaing A (1983) Stability of two Ca2+−dependent neutral proteinases and their specific inhibitor during post-mortem storage of rabbit skeletal muscle. J Sci Food Agric 34:1241–1250
Wheeler TL, Shackelford SD, Koohmaraie M (2000) Relationship of beef Longissimus tenderness classes to tenderness of Gluteus medius, semimembranosus, and Biceps femoris. J Anim Sci 78:2856–2861
Yoshimura N, Kikuchi T, Sasaki T, Kitahara A, Hatanaka M, Murachi T (1983) Two distinct Ca2+-proteases (calpain I and calpain II) purified concurrently by the same method from rat kidney. J Biol Chem 258:8883–8889
Acknowledgments
We are thankful to DST, Govt. of India, for their financial support and ICAR, New Delhi (Ministry of Agriculture, Govt. of India) for proving sufficient facilities for sample analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Ethical Approval
Approval from the Institute Animal Ethical Committee was undertaken before conducting the study.
Informed Consent
Informed consent is not applicable for the nature of this study.
Rights and permissions
About this article
Cite this article
Biswas, A.K., Kripriyalini, L., Tandon, S. et al. Simultaneous Identification of Different Domains of Calpain from Blood and Turkey Meat Samples Using Casein Zymography. Food Anal. Methods 9, 2872–2879 (2016). https://doi.org/10.1007/s12161-016-0481-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0481-y