Abstract
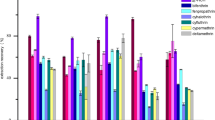
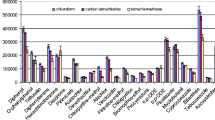
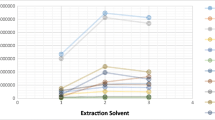
A rapid and sensitive method was established to simultaneously determine multiple pesticide residues in celery through gas chromatography-mass spectrometry (GC-MS). Samples were extracted through a modified quick, easy, cheap, effective, robust, and safe method (modified QuEChERS) and then refined and preconcentrated through dispersive liquid-liquid microextraction (DLLME) by using CHCl3 as extractive solvent and acetonitrile (ACN) as dispersive solvent. The main factors, including type of extraction solvent, volume of extraction solvent, volume of dispersive solvent, extraction time, salt concentration, vortex velocity, and pH of aqueous solution, influencing DLLME were initially evaluated by performing single-factor variable experiments; three significant factors, particularly volume of extraction solvent, volume of dispersive solvent, and extraction time, were thoroughly analyzed through response surface methodology. The following optimized extraction conditions were obtained: 100 μL of CHCl3, 900 μL of ACN, and 1.62-min extraction time. The optimized method was validated with average recoveries ranging from 70.8 to 93.2 % (with relative standard deviations of <15 %) at three spiked levels for all of the pesticides. Good linearity with determination coefficients of >0.9974 was obtained on the basis of the matrix-matched calibration curve of each pesticide; limits of detection ranging from 2.4 to 14.2 μg/kg indicated high sensitivity. Malathion with concentrations varying from 0.009 to 0.012 mg/kg was detected in all of the samples; other pesticides were not detected.







Similar content being viewed by others
References
Andraščíková M, Hrouzková S, Cunha SC (2013) Combination of QuEChERS and DLLME for GC-MS determination of pesticide residues in orange samples. Food Addit Contam A 30:286–297
Asensio-Ramos M, Hernández-Borges J, Borges-Miquel TM, Rodríguez-Delgado MÁ (2011) Ionic liquid-dispersive liquid–liquid microextraction for the simultaneous determination of pesticides and metabolites in soils using high-performance liquid chromatography and fluorescence detection. J Chromatogr A 1218:4808–4816
Berton P, Martinis EM, Wuilloud RG (2010) Development of an on-line temperature-assisted ionic liquid dispersive microextraction system for sensitive determination of vanadium in environmental and biological samples. J Hazard Mater 176:721–728
Bidari A, Ganjali MR, Norouzi P, Hosseini MRM, Assadi Y (2011) Sample preparation method for the analysis of some organophosphorus pesticides residues in tomato by ultrasound-assisted solvent extraction followed by dispersive liquid–liquid microextraction. Food Chem 126:1840–1844
Caballero-Casero N, Ocak M, Ocak Ü, Rubio S (2014) Quick supramolecular solvent-based microextraction for quantification of low curcuminoid content in food. Anal Bioanal Chem 406:2179–2187
Cunha SC, Fernandes JO (2011) Multipesticide residue analysis in maize combining acetonitrile-based extraction with dispersive liquid–liquid microextraction followed by gas chromatography–mass spectrometry. J Chromatogr A 1218:7748–7757
Di MA, Fidente P, Barbini DA, Dommarco R, Seccia S, Morrica P (2006) Application of solid-phase extraction and liquid chromatography-mass spectrometry to the determination of neonicotinoid pesticide residues in fruit and vegetables. J Chromatogr A 1108:1–6
Farajzadeh MA, Bahram M, Mehr BG, Jönsson JA (2008) Optimization of dispersive liquid–liquid microextraction of copper (II) by atomic absorption spectrometry as its oxinate chelate: application to determination of copper in different water samples. Talanta 75:832–840
Fernández M, Picó Y, Manes J (2000) Determination of carbamate residues in fruits and vegetables by matrix solid-phase dispersion and liquid chromatography–mass spectrometry. J Chromatogr A 871:43–56
Gao S, Yang X, Yu W, Liu Z, Zhang H (2012) Ultrasound-assisted ionic liquid/ionic liquid-dispersive liquid–liquid microextraction for the determination of sulfonamides in infant formula milk powder using high-performance liquid chromatography. Talanta 99:875–882
Lambropoulou DA, Albanis TA (2003) Headspace solid-phase microextraction in combination with gas chromatography-mass spectrometry for the rapid screening of organophosphorus insecticide residues in strawberries and cherries. J Chromatogr A 993:197–203
Ma H, Li Y, Zhang H, Shah SM, Chen J (2014) Salt-assisted dispersive liquid–liquid microextraction coupled with programmed temperature vaporization gas chromatography–mass spectrometry for the determination of haloacetonitriles in drinking water. J Chromatogr A 1358:14–19
Matsadiq G, Hu HL, Ren HB, Zhou YW, Liu L, Cheng J (2011) Quantification of multi-residue levels in peach juices, pulps and peels using dispersive liquid–liquid microextraction based on floating organic droplet coupled with gas chromatography-electron capture detection. J Chromatogr B 879:2113–2118
Melo A, Mansilha C, Pinho O, Ferreira IMPLVO (2013) Analysis of pesticides in tomato combining QuEChERS and dispersive liquid-liquid microextraction followed by high-performance liquid chromatography. Food Anal Method 6:559–568
Pena MT, Casais MC, Mejuto MC, Cela R (2009) Development of an ionic liquid based dispersive liquid–liquid microextraction method for the analysis of polycyclic aromatic hydrocarbons in water samples. J Chromatogr A 1216:6356–6364
Pirard C, Widart J, Nguyen BK, Deleuze C, Heudt L, Haubruge E, De Pauw E, Focant JF (2007) Development and validation of a multi-residue method for pesticide determination in honey using on-column liquid–liquid extraction and liquid chromatography–tandem mass spectrometry. J Chromatogr A 1152:116–123
Qiao F, Zhang X, Wang M, Kang Y (2010) Rapid extraction of imidacloprid in tomatoes by ultrasonic dispersion liquid–liquid microextraction coupled with LC determination. Chromatographia 72:331–335
Rezaee M, Assadi Y, Milani Hosseini MR, Aghaee E, Ahmadi F, Berijani S (2006) Determination of organic compounds in water using dispersive liquid–liquid microextraction. J Chromatogr A 1116:1–9
Wang C, Wu Q, Wu C, Wang Z (2011) Application of dispersion–solidification liquid–liquid microextraction for the determination of triazole fungicides in environmental water samples by high-performance liquid chromatography. J Hazard Mater 185:71–76
Wu C, Liu N, Wu Q, Wang C, Wang Z (2010) Application of ultrasound-assisted surfactant-enhanced emulsification microextraction for the determination of some organophosphorus pesticides in water samples. Anal Chim Acta 679:56–62
Yan H, Wang H, Qiao J, Yang G (2011) Molecularly imprinted matrix solid-phase dispersion combined with dispersive liquid–liquid microextraction for the determination of four Sudan dyes in egg yolk. J Chromatogr A 1218:2182–2188
You X, Wang S, Liu F, Shi K (2013) Ultrasound-assisted surfactant-enhanced emulsification microextraction based on the solidification of a floating organic droplet used for the simultaneous determination of six fungicide residues in juices and red wine. J Chromatogr A 1300:64–69
Zawiyah S, Che Man YB, Nazimah SAH, Chin CK, Tsukamoto I, Hamanyza AH, Norhaizan I (2007) Determination of organochlorine and pyrethroid pesticides in fruit and vegetables using sax/psa clean-up column. Food Chem 102:98–103
Zhang Y, Lee HK (2013) Low-density solvent-based vortex-assisted surfactant-enhanced-emulsification liquid–liquid microextraction combined with gas chromatography–mass spectrometry for the fast determination of phthalate esters in bottled water. J Chromatogr A 1274:28–35
Zhang S, Yang X, Yin X, Wang C, Wang Z (2012) Dispersive liquid–liquid microextraction combined with sweeping micellar electrokinetic chromatography for the determination of some neonicotinoid insecticides in cucumber samples. Food Chem 133:544–550
Zhang J, Liang Z, Guo H, Gao P, Lu R, Zhou W, Zhang S, Gao H (2013) Ionic liquid-based totally organic solvent-free emulsification microextraction coupled with high performance liquid chromatography for the determination of three acaricides in fruit juice. Talanta 115:556–562
Zhou YW, Han LT, Cheng J, Guo F, Zhi XR, Hu HL, Chen G (2011) Dispersive liquid–liquid microextraction based on the solidification of a floating organic droplet for simultaneous analysis of diethofencarb and pyrimethanil in apple pulp and peel. Anal Bioanal Chem 399:1901–1906
Zhou X, Cao S, Li X, Tang B, Ding X, Xi C, Hu J, Chen Z (2015) Simultaneous determination of 18 preservative residues in vegetables by ultra high performance liquid chromatography coupled with triplequadrupole/linear ion trap mass spectrometry using a dispersive-SPE procedure. J Chromatogr B 989:21–26
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by The National Natural Science Foundation of China (31071702).
Conflict of Interest
Yaru Wang declares that she has no conflict of interest. Xuexue Miao declares that she has no conflict of interest. Haifeng Wei declares that he has no conflict of interest. Deyun Liu declares that he has no conflict of interest. Gaofeng Xia declares that he has no conflict of interest. Xiaoyun Yang declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent is not applicable in this study.
Rights and permissions
About this article
Cite this article
Wang, Y., Miao, X., Wei, H. et al. Dispersive Liquid-Liquid Microextraction Combined with Gas Chromatography-Mass Spectrometry for the Determination of Multiple Pesticides in Celery. Food Anal. Methods 9, 2133–2141 (2016). https://doi.org/10.1007/s12161-015-0390-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-015-0390-5