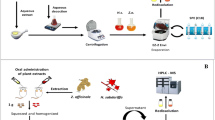
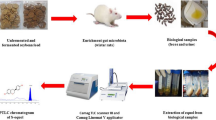
Abstract
Phytoestrogens (isoflavones, ellagitannins, and lignans) are polyphenols with an estrogenic/antiestrogenic function present in fruits, vegetables, and foods derived from them. Some microorganisms are capable of transforming isoflavones, ellagitannins, and lignans into equol, urolithins, and enterolignans, respectively. These compounds are more bioavailable, they have more estrogenic/antiestrogenic and antioxidant activity than their precursors, and they are associated with beneficial effects for human health. Extracts of soybean and lignans and ellagic acid were incubated with feces, or bacteria isolated from feces, and later the phytoestrogens were extracted twice with diethyl ether and twice with ethyl acetate, and the solvents were evaporated at room temperature under a N2. Subsequently, phytoestrogens were detected by HPLC-PAD and chromatographic peaks were identified by HPLC-ESI/M, and they were confirmed by comparison of retention times and characteristics of UV spectra with those of standards. Analysis of phytoestrogens by means of HPLC-PAD allowed the simultaneous detection of isoflavones, ellagitannins, and lignans metabolized, or not, by intestinal microbiota with high sensitivity (LOD: equol 0.11 mg/L; urolithin A 0.17 mg/L, enterodiol 0.15 mg/L). Assays were linear up to at least 20 mg/L in all standards with a high correlation (equol >0.999; urolithin A >0.998, enterodiol >0.999). Therefore, the described method constitutes an affordable and easy alternative method for the detection of phytoestrogen metabolism. Moreover, this method could be applied to the detection of isoflavones, lignans, and ellagitannins in food.





Similar content being viewed by others
References
ACS, American Chemical Society – Subcommittee on Environmental Analytical Chemistry (1980) Guidelines for data acquisition and data quality evaluation in environmental chemistry. Anal Chem 52:2242–2249
Bartkiene E, Juodeikiene G, Basinskiene L, Liukkonen K-H, Adlercreutz H, Kluge H (2011) Enterolignans enterolactone and enterodiol formation from their precursors by the action of intestinal microflora and their relationship with non-starch polysaccharides in various berries and vegetables. LWT - Food Sci Technol 44:48–53
Borges G, Roowi S, Rouanet J-M, Duthie GG, Lean MEJ, Crozier A (2007) The bioavailability of raspberry anthocyanins and ellagitannins in rats. Mol Nutr Food Res 51:714–725
Cerdá B, Llorach R, Cerón JJ, Espín JC, Tomás-Barberán FA (2003) Evaluation of the bioavailability and metabolism in the rat of punicalagin and antioxidant polyphenol from pomegranate juice. Eur J Nutr 42:18–28
Cerdá B, Espín JC, Parra S, Martínez P, Tomás-Barberán FA (2004) The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolized into bioavailable but poor antioxidant hydroxy- 6H-dibenzopyran-6-one derivatives by the colonic microflora in healthy humans. Eur J Nutr 43:205–220
Cerdá B, Periago P, Espín JC, Tomás-Barbera FA (2005) Identification of urolithin A as a metabolite produced by human colon microflora from ellagic acid and related compounds. J Agric Food Chem 53:5571–5576
Cerdá B, Soto C, Albaladeio MD, Martínez P, Sánchez-Gascón F, Tomás-Barberan FA (2006) Pomegranate juice supplementation in chronic obstructive pulmonary disease: a 5-week randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr 60:245–253
Coward L, Kirk M, Albin N, Barnes S (1996) Analysis of plasma isoflavones by reversed-phase HPLC-multiple reaction ion monitoring-mass spectrometry. Clin Chim Acta 247:121–142
Decroos K, Vanhemmens S, Cattoir S, Boon N, Verstraete W (2005) Isolation and characterisation of an equol-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions. Arch Microbiol 183:45–55
Dueñas M, Hernández T, Estrella I, Fernández D (2009) Germination as a process to increase the polyphenols content and antioxidant activity of lupin seeds (Lupinus angustifolius L.). Food Chem 117:599–607
Elghali S, Mustafa S, Amid M, Manap MYABD, Ismail A, Abas F (2012) Bioconversion of daidzein to equol by Bifidobacterium breve 15700 and Bifidobacterium longum BB536. J Funct Foods 4:736–745
Espín JC, González-Barrio R, Cerdá B, López-Bote C, Rey AI, Tomás-Barberán FA (2007) Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J Agric Food Chem 55:10476–10485
Fischer UA, Jaksch AV, Carle R, Kammerer DR (2012) Determination of lignans in edible and nonedible parts of pomegranate (Punica granatum L.) and products derived therefrom, particularly focusing on the quantitation of isolariciresinol using HPLC-DAD-ESI/MSn. J Agric Food Chem 60:283–292
Franke AA, Custer LJ, Cerna CM, Narala KK (1994) Quantitation of phytoestrogens in legumes by HPLC. J Agric Food Chem 42:1905–1913
Gavina JMA, Priem J, Wood CM, Xiao CW, Feng Y-L (2013) Determination of isoflavones in rat serum using liquid chromatography–tandem mass spectrometry with a highly efficient core–shell column. Anal Bioanal Chem 405:2643–2651
Gómez-Caravaca AM, Verardo V, Toselli M, Segura-Carretero A, Fernández-Gutiérrez A, Caboni MF (2013) Determination of the major phenolic compounds in pomegranate juices by HPLC–DAD–ESI-MS. J Agric Food Chem 61:5328–5337
Heinonen S, Nurmi T, Liukkonen K, Poutanen K, Wähälä K, Deyama T, Nishibe S, Adlercreutz H (2001) In vitro metabolism of plant lignans: new precursors of mammalian lignans enterolactone and enterodiol. J Agric Food Chem 49:3178–3186
Hendrich S (2002) Bioavailability of isoflavones. J Chromatogr B 777:203–210
Hur HG, Lay JO Jr, Beger RD, Freemanand JP, Rafii F (2000) Isolation of human intestinal bacteria metabolizing the natural isoflavone glycosides daidzin and genistin. Arch Microbiol 174:422–428
Ito H, Iguchi A, Hatano T (2008) Identification of urinary and intestinal bacterial metabolites of ellagitannin geraniin in rats. J Agric Food Chem 56:393–400
Jones AE, Price KR, Fenwick GR (1989) Development and application of a high-performance liquid chromatographic method for the analysis of phytoestrogens. J Sci Food Agric 46:357–364
Landete JM (2011) Ellagitannins, ellagic acid and their derived metabolites: a review about source, metabolism, functions and health. Food Res Int 44:1150–11609
Landete JM (2012) Plant and mammalian lignans: a review of source, intake, metabolism, intestinal bacteria and health. Food Res Int 46:410–424
Larrosa M, González-Sarrías A, García-Conesa MT, Tomás-Barberán FA, Espín JC (2006) Urolithins, ellagic acid-rerived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities. J Agric Food Chem 54:1611–1620
Masko EM, Allott EH, Freedland SJ (2013) The relationship between nutrition and prostate cancer: is more always better? Eur Urol 63:810–820
Maubach J, Bracke ME, Heyerick A, Depypere HT, Serreyn RF, Mareel MM, Keukeleire DD (2003) Quantitation of soy-derived phytoestrogens in human breast tissue and biological fluids by high-performance liquid chromatography. J Chromatogr B 784:137–144
Mukker JK, Kotlyarova V, Singh RSP, Alcorn J (2010) HPLC method with fluorescence detection for the quantitative determination of flaxseed lignans. J Chromatogr B 878:3076–3082
Possemiers S, Bolca S, Eeckhaut E, Depypere H, Verstraete W (2007) Metabolism of isoflavones, lignans and prenylflavonoids by intestinal bacteria: producer phenotyping and relation with intestinal community. FEMS Microbiol Ecol 61:372–383
Schmidt TJ, Hemmati SE, Fuss AW, Alfermann A (2006) combined HPLC-UV and HPLC-MS method for the identification of lignans and its application to the lignans of Linum usitatissimum L. and L. bienne Mill. Phytochem Anal 17:299–311
Setchell KDR, Brown NM, Zimmer-Nechemias L, Brashear WT, Wolfe BE, Kirschner AS, Heubi JE (2002) Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am J Clin Nutr 76:447–453
Smeds AI, Eklund PC, Willför SM (2012) Content, composition, and stereochemical characterisation of lignans in berries and seeds. Food Chem 134:1991–1198
Sugiyama Y, Masumori N, Fukuta F, Yoneta A, Hida T, Yamashita T, Minatoya M, Nagata Y, Mori M, Tsuji H, Akaza H, Tsukamoto T (2013) Influence of isoflavone intake and equol-producing intestinal flora on prostate cancer risk. Asian Pac J Cancer Prev 14:1–4
Touré A, Xueming X (2010) Flaxseed lignans: source, biosynthesis, metabolism, antioxidant activity, bio-active components, and health benefits. Compr Rev Food Sci Food Saf 9:261–269
Vila-Donat P, Caprioli G, Maggi F, Ricciutelli M, Torregiani E, Vittori S, Sagratini G (2015) Effective clean-up and ultra high-performance liquid chromatography–tandem mass spectrometry for isoflavone determination in legumes. Food Chem 174:487–494
Villares A, Rostagno MA, García-Lafuente A, Guillamón E, Martínez JA (2011) Content and profile of isoflavones in soy-based foods as a function of the production process. Food Bioprocess Technol 4:27–38
Vitale DC, Melilli B, Salomone S (2013) Isoflavones: estrogenic activity, biological effect and bioavailability. Eur J Drug Metab Pharmacokinet 38:15–25
Vrhovsek U, Giongo L, Mattivi F, Viola R (2008) A survey of ellagitannin content in raspberry and blackberry cultivars grown in Trentino (Italy). Eur Food Res Technol 226:817–824
Wang C, Wang L, Zhao Q, Chen J, Zheng L, Zheng M, Zhang R, Wang Z (2013) Cloud point extraction coupled with HPLC-DAD for the determination of genistein and daidzein in river water. Anal Methods 5:3688–3692
Zubik L, Meydani M (2003) Bioavailability of soybean isoflavones from aglycone and glucoside forms in American women. Am J Clin Nutr 77:1459–1465
Acknowledgments
This work was supported by RM2012-00004-00-00. J.M. Landete has a postdoctoral contract with the research program “Ramón y Cajal” (MINECO, Spain). We are grateful to the ICTAN Analysis Services Unit for providing chromatography and mass spectrometry facilities.
Conflict of Interest
Pilar Gaya declares that she has no conflict of interest. Juan Luis Arqués declares that he has no conflict of interest. Margarita Medina declares that she has no conflict of interest. Inmaculada Álvarez declares that she has no conflict of interest. José Mª Landete declares that he has no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gaya, P., Arqués, J.L., Medina, M. et al. A New HPLC-PAD/HPLC-ESI-MS Method for the Analysis of Phytoestrogens Produced by Bacterial Metabolism. Food Anal. Methods 9, 537–547 (2016). https://doi.org/10.1007/s12161-015-0226-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-015-0226-3