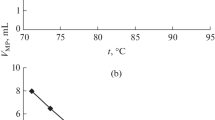
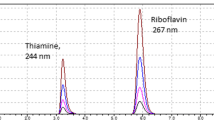
Abstract
A new micellar mediated cloud-point extraction procedure (CPE) has been developed for sensitive and selective determination of trace amounts of total iodine by means of indirect spectrophotometry. The method is based on selective charge transfer complex formation of I3, which is produced by the reaction of iodate in presence of 25-fold excess periodate with excess iodide and with acridine orange (AOH+) being a selective fluorescent cationic dye at pH 3.0 phthalate buffer, and then the CPE of charge transfer complex formed from aqueous solution at phthalate buffer using nonionic surfactant, Triton X-114. The calibration graphs were rectilinear in the ranges of 1.5–20 and 20–160 μg L−1 for IO3 −, with r 2 higher than 0.9996 in two linear regions with changing sensitivity. Under optimal conditions obtained, the limits of detection and quantification were 0.4 and 1.5 μg L−1 and the precision (as RSD) for determination of 10, 50, and 100 μg L−1 of iodate was in the range of 2.4–4.7 % (n 5). The method was successfully applied to selective and reliable determination of total iodine in milk-based nutritional products. The accuracy of the method was controlled by analyzing two standard reference materials (SRMs) at suitable matrix to milk samples.




Similar content being viewed by others
References
Altunay N, Gürkan R (2015) A new cloud point extraction procedure for determination of inorganic antimony species in beverages and biological samples by flame atomic absorption spectrometry. Food Chem 175:507–515
Altunay N, Gürkan R, Sertakan K (2015) Indirect Determination of Free, Total, and Reversibly Bound Sulfite in Selected Beverages by Spectrophotometry Using Ultrasonic-Assisted Cloud Point Extraction as a Preconcentration Step. Food Anal. Method. 1–13. doi:10.1007/s12161-015-0094-x
Anke M, Groppel B, Müller M, Scholz E, Krämer K (1995) The iodine supply of humans depending on site, food offer and water supply. Fresen J Anal Chem 352(1–2):97–101
Arena MP, Porter MD, Fritz JS (2002) Rapid, specific determination of iodine and iodide by combined solid-phase extraction/diffuse reflectance spectroscopy. Anal Chem 74(1):185–190
Chen JH, Wang KE, Jiang SJ (2007) Determination of iodine and bromine compounds in foodstuffs by CE-inductively coupled plasma MS. Electrophoresis 28(22):4227–4232
Dario Falcone R, Mariano Correa N, Alicia Biasutti M, Juana JS (2002) Acid–base and aggregation processes of acridine orange base in n-heptane/AOT/water reverse micelles. Langmuir 18:2039–2047
Das P, Gupta M, Jain A, Verma KK (2004) Single drop microextraction or solid phase microextraction–gas chromatography–mass spectrometry for the determination of iodine in pharmaceuticals, iodized salt, milk powder and vegetables involving conversion into 4-iodo-N. N-dimethylaniline J Chromatogr A 1023:33–39
Department of Health (1991) Report of the panel on dietary reference values of the Committee on Medical Aspects of Food Policy (COMA). In: Dietary Reference Values for Food Energy and Nutrients for the United Kingdom. London: The Stationery Office.
Dutta A, Dutta RK (2013) Protonation of acridine orange in dye-surfactant ion pair micelles. J Mol Liq 178:25–30
El Walily AFM, El Gindy A, Wahbi AAM (1995) Spectrophotometric determination of flunarizine dihydrochloride through the formation of charge transfer complex with iodine. J Pharmaceut Biomed 13(1):53–58
Fassett JD, Murphy TJ (1990) Determination of iodine in oyster tissue by isotope dilution laser resonance ionization mass spectrometry. Anal Chem 62(4):386–389
Guocheng L, Zhaohui L, Wei-Teh J, Po-Hsiang C, Jin-Shuh J, Kao-Hung L (2011) Mechanism of acridine orange removal from water by low-charge swelling clays. Chem Eng J 174:603–611
Gupta M, Pillai AK, Singh A, Jain A, Verma KK (2011) Salt-assisted liquid–liquid microextraction for the determination of iodine in table salt by high-performance liquid chromatography-diode array detection. Food Chem 124(4):1741–1746
Gürkan R, Yılmaz Ö (2013) Indirect quantification of trace levels cyanide in environmental waters through flame atomic absorption spectrometry coupled with cloud point extraction. J Iranian Chem Soc 10(4):631–642
Gürkan R, Kır U, Altunay N (2015) Development of a simple, sensitive and inexpensive ion-pairing cloud point extraction approach for the determination of trace inorganic arsenic species in spring water, beverage and rice samples by UV–Vis spectrophotometry. Food Chem 180:32–41
Han X, Cao L, Cheng H, Liu J, Xu Z (2012) Determination of iodine species in seaweed and seawater samples using ion-pair reversed phase high performance liquid chromatography coupled with inductively coupled plasma mass spectrometry. Anal Met 4(10):3471–3477
Hu M, Chen H, Jiang Y, Huifang Zhu H (2013) Headspace single-drop microextraction coupled with gas chromatography electron capture detection of butanone derivative for determination of iodine in milk powder and urine. Chem Pap 67(10):1255–1261
Huynh D, Zhou SJ, Gibson R, Palmer L, Muhlhausler B (2015) Validation of an optimized method for the determination of iodine in human breast milk by inductively coupled plasma mass spectrometry (ICP-MS) after tetramethylammonium hydroxide extraction. J Trace Elem Med Biol 29:75–82
Isaac-Olive K, Chatt A (2012) Studies of total, organic and inorganic iodine in Canadian bovine milk samples with varying milk fat content using ion-exchange chromatography and neutron activation analysis. J Radioanal Nucl Chem 294(3):479–486
Komaromy-Hiller G, Calkins N, Wandruszka R (1996) Changes in polarity and aggregation number upon clouding of a nonionic detergent: effect of ionic surfactants and sodium chloride. Langmuir 12:916–920
Lucia C, Gennaro G, Ornella O, Vincenzo V (1984) Acridine orange association equilibrium in aqueous solution. J Chem Eng Data 29(1):62–66
Mesko MF, Mello PA, Bizzi CA, Dressler VL, Knapp G, Flores ÉM (2010) Iodine determination in food by inductively coupled plasma mass spectrometry after digestion by microwave-induced combustion. Anal Bioanal Chem 398(2):1125–1131
Murad MM (1999) Fluorescence analysis of acridine orange adsorbate at the water/N-heptane interface, bulk and interface. J Fluoresc 9(3):257–263
Nascentes CC, Arruda MAZ (2003) Cloud point formation based on mixed micelles in the presence of electrolytes for cobalt extraction and preconcentration. Talanta 61(6):759–768
Pena-Pereira F, Lavilla I, Bendicho C (2009) Headspace single-drop microextraction coupled to microvolume UV–vis spectrophotometry for iodine determination. Anal Chim Acta 631(2):223–228
Preedy V.R, Burrow G.N, Watson R (2009) Comprehensive handbook of iodine: nutritional, biochemical, pathological and therapeutic aspects. Comprehensive handbook of iodine: nutritional, biochemical, pathological and therapeutic aspects. Academic Press, Amsterdam.
Reid HJ, Bashammakh AA, Goodall PS, Landon MR, O’Connor C, Sharp BL (2008) Determination of iodine and molybdenum in milk by quadrupole ICP-MS. Talanta 75(1):189–197
Saleh GA, Askal HF, Radwan MF, Omar MA (2001) Use of charge transfer complexation in the spectrophotometric analysis of cetain cephalosporins. Talanta 54(6):1205–1215
Sharma VK, Sahare PD, Rastogi RC, Ghoshal SK, Mohan D (2003) Excited state characteristics of acridine dyes: acriflavine and acridine orange. Spectochim Acta A 59:1799–1804
Shinoda T, Miyamoto N, Kuromoto T, Ito K, Morikawa H, Okamoto Y, Hirokawa T (2012) Pyrohydrolysis coupled to ion chromatography for sensitive determination of iodine in food-related materials. Anal Let 45(8):862–871
Ulusoy HI, Aksoy UG, Akcay M (2013) Simultaneous pre-concentration of Pb and Sn in food samples and determination by atomic absorption spectrometry. Eur Food Res Technol 236(4):725–733
Varga I (2007) Iodine determination in dietary supplement products by TXRF and ICP-OES. Microchem J 85(1):127–131
WHO (World Health Organization) (1995) Reliable evaluation of low-level contamination of food-Workshop in the frame of GEMS/food-EURO. Federal Republic of Germany, Kulmbach
WHO (World Health Organization) (2004) Iodine status worldwide WHO global database on iodine deficiency. Geneva, World Health Organization, Department of Nutrition for Health and Development: 48.
Yazdi AS, Yazdinezhad SR, Akhoundzadeh J (2013) Simultaneous derivatization and extraction of iodine from milk samples by hollow fiber liquid-phase microextraction followed by gas chromatography-electron capture detection. J Iranian Chem Soc 10(4):643–651
Yoshida S, Muramatsu Y, Katou S, Sekimoto H (2007) Determination of the chemical forms of iodine with IC-ICP-MS and its application to environmental samples. J Radioanal Nucl Ch 273(1):211–214
Zimmermann MB (2009) Iodine deficiency in pregnancy and the effects of maternal iodine supplementation on the offspring: a review. Am J Clin Nutr 89(2):668S–672S
Acknowledgments
Financial assistance from the Cumhuriyet University Scientific Research Projects Commission (CUBAP), Sivas in Turkey, is sincerely thanked. Thanks are also due to Prof. Dr. Mehmet Akçay for his meaningful helps and contributions in critical evaluation and publication step of the presented research article.
Conflict of Interests
The authors declare that they have no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
✓ The indirect CPE preconcentration method prior to detection of total iodine is proposed.
✓ The method is based on ion-pairing of I3 − to AOH+ in presence of KCl as salting out agent at pH 3.0.
✓ It has a detection limit of 0.4 μg L−1 with a good sensitivity improvement in range of 1.5–160 μg L−1.
✓ It was successfully applied to quantification of total iodine in milk based nutritional products.
✓ It is an accurate and reliable method to monitor trace iodate in presence of excess periodate.
Rights and permissions
About this article
Cite this article
Altunay, N., Gürkan, R. A New Micellar Mediated Cloud-Point Extraction Procedure for Sensitive and Selective Determination of Trace Amounts of Total Iodine in Milk-Based Nutritional Products by Means of Indirect Spectrophotometry. Food Anal. Methods 9, 505–518 (2016). https://doi.org/10.1007/s12161-015-0220-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-015-0220-9