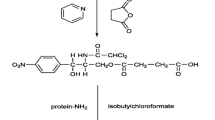
Abstract
An analytical method to determine antibiotics using a lab-built drop-type chemiluminescence (DCL) system is introduced. Magnetic nanoparticles (MNPs) functionalized with amine groups were used for the extraction of antibiotics from a pretreated sample solution by magnetic separation. Detection is achieved with an antibody specific against enrofloxacin and ciprofloxacin and a secondary horseradish peroxidase (HRP)-labelled antibody. The enzyme is capable of catalyzing the luminol reaction, which can be read out with the DCL device. For application, the DCL system was used to determine enrofloxacin and its metabolite in chicken meat. The obtained detection limits were 0.039 pmol/mL (~0.12 ng/mL) and 0.022 pmol/mL for enrofloxacin and ciprofloxacin, respectively, which are about 10 times better than enzyme-linked immunosorbent assay (ELISA).




Similar content being viewed by others
References
Andreu A, Blasco C, Picó Y (2007) Analytical strategies to determine quinolone residues in food and the environment. TrAC Trends Anal Chem 26:534–556
Chen J, Xu F, Jiang H, Hou Y, Rao Q, Guo P, Ding S (2009) A novel quantum dot-based fluoroimmunoassay method for detection of enrofloxacin residue in chicken muscle tissue. Food Chem 113:1197–1201
Chen F, Lin Z, Zheng Y, Zeng H, Nakajima H, Uchiyama K, Lin JM (2012) Development of an automatic multi-channel ink-jet ejection chemiluminescence system and its application to the determination of horseradish peroxidase. Anal Chim Acta 739:77–82
Cinquina AL, Roberti P, Giannetti L, Longo F, Draisci R, Fagiolo A, Brizioli NR (2003) Determination of enrofloxacin and its metabolite ciprofloxacin in goat milk by high-performance liquid chromatography with diode-array detection: optimization and validation. J Chromatogr A 987:221–226
Council Regulation (ECC) (1990) Community procedure for the establishment of maximum residue limits of veterinary medicinal products in foodstuffs of animal origin. No 2377/90 of 26 June 1990, European Union
Gratacós-Cubarsí M, García-Regueiro JA, Castellari M (2007) Assessment of enrofloxacin and ciprofloxacin accumulation in pig and calf hair by HPLC and fluorimetric detection. Anal Bioanal Chem 387:1991–1998
Huet AC, Charlier C, Tittlemier SA, Singh G, Benrejeb S, Delahaut P (2006) Simultaneous determination of (fluoro)quinolone antibiotics in kidney, marine products, eggs, and muscle by enzyme-linked immunosorbent assay (ELISA). J Agric Food Chem 54:2822–2827
Jang JH, Lim HB (2010) Characterization and analytical application of surface modified magnetic nanoparticles. Microchem J 94:148–158
Kennedy DG, Cannavan A, McCracken RJ (2000) Regulatory problems caused by contamination, a frequently overlooked cause of veterinary drug residues. J Chromatogr A 882:37–52
Kim S, Ko J, Lim HB (2013) Application of magnetic and core-shell nanoparticles to determine enrofloxacin and its metabolite using laser induced fluorescence microscope. Anal Chim Acta 771:37–41
Kim R, Sung YI, Lee JS, Lim HB (2010) Chemiluminescence system for direct determination and mapping of ultra-trace metal impurities on a silicon wafer. Analyst 135:2901–2906
Kim BS, Kim JN, Yoon SH, Chun J, Cerniglia CE (2012) Impact of enrofloxacin on the human intestinal microbiota revealed by comparative molecular analysis. Anaerobe 18:310–320
Li T, Wang E, Dong S (2008) Chemiluminescence thrombin aptasensor using high-activity DNAzyme as catalytic label. Chem Comm 43:5520–5522
Liu H, Fu Z, Yang Z, Yan F, Ju H (2008) Sampling-resolution strategy for one-way multiplexed immunoassay with sequential chemiluminescent detection. Anal Chem 80:5654–5659
Navalón A, Blanc R, Reyes L, Navas N, Vílchez JL (2002) Determination of the antibacterial enrofloxacin by differential-pulse adsorptive stripping voltammetry. Anal Chim Acta 454:83–91
Pulgarin JAM, Molina AA, Fernandez LM (2008) Determination of ciprofloxacin, the major metabolite of enrofloxacin, in milk by isopotential fluorimetry. J Agric Food Chem 56:8838–8843
Stubbings G, Bigwood T (2009) The development and validation of a multiclass liquid chromatography tandem mass spectrometry (LC–MS/MS) procedure for the determination of veterinary drug residues in animal tissue using a QuEChERS (QUick, Easy, CHeap, Effective, Rugged and Safe) approach. Anal Chim Acta 637:68–78
Tang Q, Yang T, Tan X, Luo J (2009) Simultaneous determination of fluoroquinolone antibiotic residues in milk sample by solid-phase extraction–liquid chromatography–tandem mass spectrometry. J Agric Food Chem 57:4535–4539
Wang L, Wu X, Xie Z (2005) Determination of enrofloxacin and its metabolite ciprofloxacin by high performance capillary electrophoresis with end-column amperometric detection. J Sep Sci 28:1143–1148
Wang L, Yang P, Li Y, Chen H, Li M, Luo F (2007) A flow injection chemiluminescence method for the determination of fluoroquinolone derivative using the reaction of luminol and hydrogen peroxide catalyzed by gold nanoparticles. Talanta 72:1066–1072
Wolyniec E, Karpinska J, Losiewska S, Turkowicz M, Klimczuk J, Kojlo A (2012) Determination of lipoic acid by flow-injection and high-performance liquid chromatography with chemiluminescence detection. Talanta 96:223–229
Xin TB, Wang X, Jin H, Liang SX, Lin JM, Li ZJ (2009) Development of magnetic particle-based chemiluminescence enzyme immunoassay for the detection of 17β-estradiol in environmental water. Appl Biochem Biotechnol 158:582–594
Yang Z, Fu Z, Yan F, Liu H, Ju H (2008) A chemiluminescent immunosensor based on antibody immobilized carboxylic resin beads coupled with micro-bubble accelerated immunoreaction for fast flow-injection immunoassay. Biosens Bioelectron 24:35–40
Yang M, Kostov Y, Bruck HA, Rasooly A (2009) Gold nanoparticle-based enhanced chemiluminescence immunosensor for detection of staphylococcal enterotoxin B (SEB) in food. Int J Food Microbiol 133:265–271
Yang C, Zhang Z, Shi Z (2010) A novel chemiluminescence reaction system for the determination of lincomycin with diperiodatonickelate (IV). Microchim Acta 168:293–298
Yorke JC, Froc P (2000) Quantitation of nine quinolones in chicken tissues by high-performance liquid chromatography with fluorescence detection. J Chromatogr A 882:63–77
Zhao Y, Zhang G, Liu Q, Teng M, Yang J, Wang J (2008) Development of a lateral flow colloidal gold immunoassay strip for the rapid detection of enrofloxacin residues. J Agric Food Chem 56:12138–12142
Acknowledgments
This work was supported by the Korea Food and Drug Administration in 2012 (10162KFDA995).
Conflict of Interest
H. B. Lim declares that he has no conflict of interest. Jungwon Ahn declares that she has no conflict of interest.
Compliance with Ethics Requirements
For demonstration of the developed method in this work, chicken meat treated for food was purchased from local food market. All institutional and national guidelines for the care and use of laboratory animals were followed.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PPTX 611 kb)
Rights and permissions
About this article
Cite this article
Ahn, J., Lim, H.B. Drop-Type Chemiluminescence (DCL) System and Sample Treatment Platform Using Magnetic Nanoparticles to Determine Enrofloxacin and Its Metabolite in a Chicken Meat. Food Anal. Methods 8, 79–85 (2015). https://doi.org/10.1007/s12161-014-9871-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-014-9871-1