Abstract
In the current study, a new cloud point extraction (CPE) procedure was developed for the separation and preconcentration of inorganic soluble As species, As(III), As(V), and total As in water and beverage samples. Selective ion-pairing complex of As(III) with Neutral red (NRH+) being a cationic phenazine-based dye in presence of citric acid at pH 2.0 was extracted into the surfactant-rich phase of octylphenoxypolyethoxyethanol (Triton X-114) from samples. After phase separation, the preconcentrated As(III) was determined by means of spectrophotometer at 542 nm. After optimization of the CPE conditions, a preconcentration factor of 50 and the detection and quantification limits of 1.44 and 4.8 μg L−1 with a correlation coefficient of 0.9953 were obtained from the calibration curve constructed in the range of 5–1500 μg L−1 for As(III). The precision of the method (as RSD) was in the range of 2.2–4.5 % (25, 100, and 750 μg L−1, N = 5). The As(V) contents of samples were calculated from the difference between As(III) and total As contents after the reduction of As(V) to As(III) with mixture of KI and ascorbic acid at HCl media. The method is very versatile and inexpensive because it exclusively used conventional UV–Vis spectrophotometry. The method was succesfully applied to the simultenous determination of inorganic arsenic species in different water and beverage samples. Its accuracy and precision were controlled by analysis of two certified reference materials (CRMs).

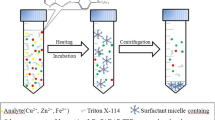
Experimental steps of the CPE procedure for detection of As(III), As(V), and total As in beverage samples.







Similar content being viewed by others
References
Abbas A, Tayyebeh M, Akbar AA (2001) Kinetic-spectrophotometric determination of trace amounts of As (III) based on its inhibitory effect on the redox reaction between bromate and hydrochloric acid. Talanta 55:55–60
Adriano DC (2001) Metals in the Terrestrial Environment. Springer, New York
Amjadi M, Manzoori JL, Taleb Z (2010) Reverse micelle coacervate-based extraction combined with electrothermal atomic absorption spectrometry for the determination of arsenic in water and oyster tissue samples. Microchim Acta 169:187–193
Azhar AG, Zuhair AAK, Kasim HK (2014) Selective extraction and determination of arsenic (III) and arsenic (V) in some food samples by cloud-point extraction coupled with hydride generation atomic absorption spectrometry. Int Res J Pure Appl Chem 4(3):362–377
Barros H, Parra LMM, Bennun L, Greaves ED (2010) Determination of arsenic in water samples by total reflection x-ray fluorescence using pre-concentration with alumina. Spectrochim Acta Part B 65:489–492
Carabias-Martinez R, Rodrıguez-Gonzalo E, Dominguez-Alvarez J, Hernández-Méndez J (1999) Cloud point extraction as a preconcentration step prior to capillary electrophoresis. Anal Chem 71(13):2468–2474
Carvalho JM, Leandro KC (2012) Determination of As (III) and As (V) in grape juice samples by differential pulse cathodic stripping voltammetry. Rev Inst Adolfo Lutz 71(1):100–104
Chand P, Badiadka N (2008) Determination of arsenic in environmental and biological samples using toluidine blue or safranine O by simple spectrophotometric method. Bull Environ Contam Toxicol 81:47–51
Coelho NMM, Carmen P, Cervera ML, Pastor A, Guardia M (2003) High performance liquid chromatography—atomic fluorescence spectrometric determination of arsenic species in beer samples. Anal Chim Acta 482:73–80
Coelho NMM, Coelho LM, Lima ES, Pastor A, Guardia M (2005) Determination of arsenic compounds in beverages by high-performance liquid chromatography-inductively coupled plasma mass spectrometry. Talanta 66:818–822
Din MI, Ata S, Mohsin IU, Rasool A, Aziz A (2014) Evaluation of conductive polymers as an adsorbent for eradication of As (III) from aqueous solution using inductively coupled plasma optical emission spectroscopy (ICP-OES). Int J Sci Eng 6(2):154–162
EFSA (European Food Safety Authority) (2009) Scientific Opinion on Arsenic in Food; EFSA Panel on Contaminants in the Food Chain. The EFSA Journal 7:(10):1351 EFSA, Parma, Italy
Fang D, Ruizhi D, Kai Y, Xubiao L, Xinman T, Shenglian L, Lixia Y (2013) Determination of trace total inorganic arsenic by hydride generation atomic fluorescence spectrometry after solid phase extraction-preconcentration on aluminium hydroxide gel. Microchim Acta 180:509–515
Gabriela B, Tatiana VS, Martin K, Radko V, Vladimír K (2008) Optimalization of poly (Neutral Red) coated- wire electrode for determination of citrate in soft drinks. Sensors 8:594–606
Henze G, Wolfram W, Sylvia S (1997) Speciation of arsenic(V) and arsenic(III) by cathodic stripping voltammetry in fresh water samples. Fresenius J Anal Chem 358:741–744
Hsieh YJ, Jiang SJ (2012) Application of HPLC-ICP-MS and HPLC-ESI-MS procedures for arsenic speciation in seaweeds. J Agric Food Chem 60:2083–2089
Hsieh MW, Liu CL, Chen JH, Jiang SJ (2010) Speciation analysis of arsenic and selenium compounds by CE-dynamic reaction cell-ICP-MS. Electrophoresis 13:2272–2278
Igov AR, Simonovi RM, Igov R (2003) Kinetic determination of ultramicro amounts of As(III) in solution. J Serb Chem Soc 68:131–135
Ioannis NP, Nikolaos ST, Efrosini AP (2013) Determination of total arsenic, total inorganic arsenic and inorganic arsenic species in rice and rice flour by electrothermal atomic absorption spectrometry. Microchem J 108:1–6
Izabela K, Danuta B (2011) Arsenic and its speciation in water samples by high performance liquid chromatography inductively coupled plasma mass spectrometry—Last decade review. Talanta 84:247–261
Junling Z, Gang Z, Chengji Z, Xinjun Q, Qiong J (2012) On-line preconcentration/separation of inorganic arsenic and antimony by poly (aryl ether ketone) containing pendant carboxyl groups prior to microwave plasma atomic spectrometry determinations. Microchem J 100:95–99
Khley C, Kihwan C, Jihye K, In HS, Doo SC (2013) Sensitive arsenic analysis by carrier-mediated counter-transport single drop microextraction coupled with capillary electrophoresis. Microchem J 106:220–225
Kuenstl L, Griesel S, Prange A, Goessler W (2009) Arsenic speciation in bodily fluids of harbor seals (Phoca vitulina) and harbor porpoises (Phocoena phocoena). Environ Chem 6:319–327
Lan Y, Luo H, Ren X, Wang Y, Liu Y (2012) Anodic stripping voltammetric determination of arsenic(III) using a glassy carbon electrode modified with gold-palladium bimetallic nanoparticles. Microchim Acta 178:153–161
Leticia BE, Estefanía MM, Roberto AO, Rodolfo GW (2013) Arsenic speciation analysis in mono-varietal wines by on-line ionic liquid-based dispersive liquid–liquid microextraction. Food Chem 138:484–490
Maja W, Wieslaw Z (2014) Optimization of sample preparation of carrot-fruit juice for determination of antimony, arsenic, and selenium by hydride generation - ınductively coupled plasma optical emission spectrometry. Anal Lett 47:2104-2119
Mehdi A, Nazly N, Hamed T, Alireza H, Parvaneh R, Mehrnoush M, Reza F (2014) Application of multi-factorial experimental design to successfully model and optimize inorganic arsenic speciation in environmental water samples by ultrasound assisted emulsification of solidified floating organic drop microextraction. Anal Method 6:2973–2981
Moreira CM, Duarte FA, Julia L, Dirce P, Flores MM, Dressler VL (2011) Arsenic speciation in white wine by LC–ICP–MS. Food Chem 126:1406–1411
Moreno E, Camara C, Corns WT, Bryce DW, Stockwell PB (2000) Arsenic speciation in beverages by direct injection-ion chromatography-hydride generation atomic fluorescence spectrometry. J Autom Methods Manag Chem 22:33–39
Murata R, Shimizu N, Uehara T (2005) Speciation arsenic(III) and arsenic(V) in natural water by graphite furnace AAS after coprecipitation with a copper‐pyrrolidinedithiocarbamate complex. Bunseki Kagaku 54:831–836
Sasan R, Mozhgan B, Britta PF (2013) Speciation of arsenite and arsenate by electrothermal AAS following ionic liquid dispersive liquid-liquid microextraction. Microchim Acta 180:415–421
Sorbo A, Turco AC, Di Gregorio M, Ciaralli L (2014) Development and validation of an analytical method for the determination of arsenic, cadmium and lead content in powdered infant formula by means of quadrupole inductively coupled plasma mass spectrometry. Food Control 44:159–165
Tang AN, Ding GS, Yan XP (2005) Cloud point extraction for the determination of As(III) in water samples by electrothermal atomic absorption spectrometry. Talanta 67:942–946
Tomoko O, Jun Y, Hiroaki T, Tetsuya N (2014) Inorganic arsenic in the japanese diet: daily intake and source. Arch Environ Contam Toxicol 66:100–112
TS 266 (Turkish Standard) (2005) Water intended for human consumption (in Turkish), Ankara
Tuzen M, Uluozlu OD, Mendila D, Soylak M (2010) Determination of As(III) and As(V) species in some natural water and food samples by solid-phase extraction on Streptococcus pyogenesimmobilized on Sepabeads SP 70 and hydride generation atomic absorption spectrometry. Food Chem Toxicol 48:1393–1398
Ulusoy HI, Akcay M, Gürkan R (2011a) Development of an inexpensive and sensitive method for the determination of low quantity of arsenic species in water samples by CPE–FAAS. Talanta 85:1585–1591
Ulusoy HI, Akcay M, Ulusoy S, Gürkan R (2011b) Determination of ultra trace arsenic species in water samples by hydride generation atomic absorption spectrometry after cloud point extraction. Anal Chim Acta 703:137–144
Waiky W, Wonga K, Stephen W, Chung C, Benny TP, Chan BT, Ho YY, Ying X (2013) Dietary exposure to inorganic arsenic of the Hong Kong population: Results of the first Hong Kong Total Diet Study. Food Chem Toxicol 51:379–385
WHO (2008) Guidelines for drinking water quality, volume1, recommendation. World Health Organization, Geneva
Yin XB (2004) On-line preconcentration for capillary electrophoresis-atomic fluorescence spectrometric determination of arsenic compounds. Electrophoresis 25:1837–1842
Yinyan J, Yiwei W, Jungfeng L, Xinquan X, Danlei W (2008) Ammonium pyrrolidinedithiocarbamate-modified activated carbon micro-column extraction for the determination of As(III) in water by graphite furnace atomic absorption spectrometry. Microchim Acta 161:137–142
Zhan S, Yu M, Jing L, Lumei WP (2013) Colorimetric detection of trace arsenic(III) in aqueous solution using arsenic aptamer and gold nanoparticles. Aust J Chem 67:813–818
Acknowledgments
This study has been supported by Cumhuriyet University Scientific Research Projects Commission as the research projects with the F-417 code. Also, authors wish to acknowledge Prof. Dr. Mehmet AKÇAY for his expert discussions in the preparation of this manuscript.
Conflict of Interest
None.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• The CPE was coupled with spectrophotometry for detection of inorganic As(III)ion in beverages.
• The method is based on selective ion-pairing of As(III) with NRH+ in presence of citric acid at pH 2.0.
• The As(III) was chosen as analyte in analysis of beverages due to its selectivity and sensitivity.
• The analytical variables affecting CPE efficiency were optimized.
• A good detection limit for As(III) (1.44 μg L−1) in linear range of 5–1500 μg L−1 was achieved.
• The study can also be extended to the other matrices and possible sources of contamination.
Rights and permissions
About this article
Cite this article
Gürkan, R., Kir, U. & Altunay, N. A Novel Preconcentration Procedure Using Neutral Red as Ion-Pairing Reagent for Determination of Inorganic Dissolved Arsenic Species in Different Water and Beverages by Spectrophotometry. Food Anal. Methods 8, 1637–1651 (2015). https://doi.org/10.1007/s12161-014-0039-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-014-0039-9