Abstract
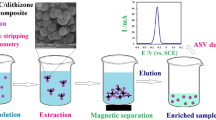
New functionalized magnetic nanoparticles as solid-phase sorbent were prepared and investigated for extraction of ultra-trace amounts of mercury from environmental samples. The Fe3O4 magnetic nanoparticles functionalized with dithizone were characterized by Fourier transform infrared spectrometer. X-ray diffraction and scanning electron microscopy confirmed the size of nanoparticles. Effects of several factors on the extraction procedure were investigated. The optimized conditions were established to be 80 mg of polymer, 8.5 for solution pH, 5 min for adsorption time, 5 min for desorption time, 2 mL for HCl (0.1 mol L−1)/ thiourea 0.05 % as the eluent, 500 mL for breakthrough volume, and without addition of salt. Under the optimal conditions, the limit of detection, maximum capacity, and preconcentration factor were 0.05 ng mL−1, 0.557 mmol g−1, and 250, respectively. Limit of quantification was in the range of 0.2–2 ng mL−1 for various matrices. Accuracy and precision of the method were about ±2.0 and below 11.1 %, respectively. Finally, the present method has been successfully applied to mercury determination in table salt, green tea, vegetables, toothpaste, and water samples. The mercury content found in the real samples was from 0.6 to 15.74 ng mL−1 without addition of mercury.






Similar content being viewed by others
References
Amini N, Cardwell TJ, Cattrall RW, Morrison RJS, Kolev SD (2004) On-line determination of mercury(II) by membrane separation flow injection analysis. Talanta 63:1069
Aranda PR, Gil RA, Moyano S, De Vito IE, Martinez LD (2008) Cloud point extraction of mercury with PONPE 7.5 prior to its determination in biological samples by ETAAS. Talanta 75:307
Ashkenani H, Dadfarnia S, Haji Shabani AM, Jaffari AA, Behjat A (2009) Preconcentration speciation and determination of ultra trace amounts of mercury by modified octadecyl silica membrane disk/electron beam irradiation and cold vapor atomic absorption spectrometry. J Hazard Mater 161:276
Atia AA, Donia AM, Al-Amrani WA (2009) Effect of amine type modifier on the uptake behavior of silica towards mercury(II) in aqueous solution. Desalination 246:257
Baghdadi M, Shemirani F (2008) Cold-induced aggregation microextraction: a novel sample preparation technique based on ionic liquids. Anal Chim Acta 613:56
Bagheri H, Naderi M (2009) Immersed single-drop microextraction–electrothermal vaporization atomic absorption spectroscopy for the trace determination of mercury in water samples. J Hazard Mater 165:353
Balarama Krishna MV, Ranjit M, Karunasagar D, Arunachalam J (2005) A rapid ultrasound-assisted thiourea extraction method for the determination of inorganic and methyl mercury in biological and environmental samples by CVAAS. Talanta 67:70
Benoit JM, Gilmour CC, Mason RP, Heyes A (1999) Sulfide controls on mercury speciation and bioavailability to methylating bacteria in sediment pore waters. Environ Sci Technol 33:951
Berzas Nevado JJ, Rodriguez Martin-Doimeadios RC, Guzman Bernardo FJ, Jimenez Moreno M (2005) Determination of mercury species in fish reference materials by gas chromatography-atomic fluorescence detection after closed-vessel microwave-assisted extraction. J Chromatogr A 1093:21
Black FJ, Bruland KW, Russell Flegal A (2007) Competing ligand exchange-solid phase extraction method for the determination of the complexation of dissolved inorganic mercury (II) in natural waters. Anal Chim Acta 598:318
Blue LY, Jana P, Atwood DA (2010) Aqueous mercury precipitation with the synthetic dithiolate, BDTH2. Fuel 89:1326
Chen J, Chen H, Jin X, Chen H (2009a) Determination of ultra-trace amount methyl-, phenyl- and inorganic mercury in environmental and biological samples by liquid chromatography with inductively coupled plasma mass spectrometry after cloud point extraction preconcentration. Talanta 77:1381
Chen H, Chen J, Jin X, Wei D (2009b) Determination of trace mercury species by high performance liquid chromatography–inductively coupled plasma mass spectrometry after cloud point extraction. J Hazar Mater 172:1282
Cheng G, He M, Peng H, Hu B (2012) Dithizone modified magnetic nanoparticles for fast and selective solid phase extraction of trace elements in environmental and biological samples prior to their determination by ICP OES. Talanta 88:507
Dakova I, Karadjova I, Georgieva V (2009) Ion-imprinted polymethacrylic microbeads as new sorbent for preconcentration and speciation of mercury. Talanta 78:523
Dittert IM, Maranhao TA, Borges DLG, Vieira MA, Welz B, Curtius AJ (2007) Determination of mercury in biological samples by cold vapor atomic absorption spectrometry following cloud point extraction with salt-induced phase separation. Talanta 72:1786
EPA National primary drinking water regulations (2002) 40 CFR Ch.I (7-1-02 ed.): website: http://www.access.gpo.gov/nara/cfr/waisidx-02/40cfr14102.html
Fan Z (2006) Hg(II)-imprinted thiol-functionalized mesoporous sorbent microcolumn preconcentration of trace mercury and determination by inductively coupled plasma optical emission spectrometry. Talanta 70:1164
Fan J, Wu C, Wei Y, Peng C, Peng P (2007) Preparation of xylenol orange functionalized silica gel as a selective solid phase extractor and its application for preconcentration-separation of mercury from waters. J Hazard Mater 145:323
Fan J, Qin Y, Ye C, Peng P, Wu C (2008) Preparation of diphenylcarbazone-functionalized silica gel and its application to on-line selective solid-phase extraction and determination of mercury by flow-injection spectrophotometry. J Hazard Mater 150:343
Faraji M, Yamini Y, Rezaee M (2010) Extraction of trace amounts of mercury with sodium dodecyl sulphate-coated magnetite nanoparticles and its determination by flow injection inductively coupled plasma-optical emission spectrometry. Talanta 81:831
Gao R, Hu Z, Chang X, He Q, Zhang L, Tu Z, Shi J (2009) Chemically modified activated carbon with 1-acylthiosemicarbazide for selective solid-phase extraction and preconcentration of trace Cu(II), Hg(II) and Pb(II) from water samples. J Hazard Mater 172:324
Garrido M, Nezio MSD, Lista AG, Palomeque M, Fernandez Band BS (2004) Cloud-point extraction/preconcentration on-line flow injection method for mercury determination. Anal Chim Acta 502:173
Giacomino A, Abollino O, Malandrino M, Mentasti E (2008) Parameters affecting the determination of mercury by anodic stripping voltammetry using a gold electrode. Talanta 75:266
Grotti M, Lagomarsino C, Magi E (2006) Simultaneous determination of arsenic, selenium and mercury in foodstuffs by chemical vapor generation inductively coupled plasma optical emission spectroscopy. Ann Chim 96:751
Hashemi P, Rahimi A (2007) A highly sensitive method for the determination of mercury using vapor generation gold wire microextraction and electrothermal atomic absorption spectrometry. Spectrochim Acta B 62:423–428
Hinds MW (1998) Determination of mercury in gold bullion by flame and graphite furnace atomic absorption spectrometry. Spectrochim Acta B 53:1063
Huang C, Hu B (2008) Silica-coated magnetic nanoparticles modified with @c-mercaptopropyltrimethoxysilane for fast and selective solid phase extraction of trace amounts of Cd, Cu, Hg, and Pb in environmental and biological samples prior to their determination by inductively coupled plasma. Spectrochim Acta B 63:437
Issaro N, Abi-Ghanem C, Bermond A (2009) Fractionation studies of mercury in soils and sediments: a review of the chemical reagents used for mercury extraction. Anal Chim Acta 631:1
Ito R, Kawaguchi M, Sakui N, Honda H, Okanouchi N, Saito K, Nakazawa H (2008) Mercury speciation and analysis in drinking water by stir bar sorptive extraction with in situ propyl derivatization and thermal desorption–gas chromatography–mass spectrometry. J Chromatogr A 1209:267
Kurmaz VA, Ershler AB (2006) Acid-catalysed degradation of the organomercury intermediates of pentafluorophenyl mercury bromide reduction near a mercury electrode. Mendeleev Commun 16:234
Lakhani SR (2007) DABTZ as a new regent for solid phase extraction and spectrophotometric determination of trace amount of Hg(II) in water sample. Ann Chim 97:9
Lalor G, DeSilva N, Rattray R, Wright PRD (2005) Design and characterisation of a vapor generation system for determination of mercury by atomic absorption spectrometry. J Anal At Spectrom 20:659
Li Y, Hu B (2007) Sequential cloud point extraction for the speciation of mercury in seafood by inductively coupled plasma optical emission spectrometry. Spectrochim Acta B 62:1153
Li Z, Wei Q, Yuan R, Zhou X, Liu H, Shan H, Song Q (2007) A new room temperature ionic liquid 1-butyl-3-trimethylsilylimidazolium hexafluorophosphate as a solvent for extraction and preconcentration of mercury with determination by cold vapor atomic absorption spectrometry. Talanta 71:68
Liu Y, Changa X, Yang D, Guob Y, Meng S (2005) Highly selective determination of inorganic mercury (II) after preconcentration with Hg(II)-imprinted diazoaminobenzene-vinylpyridine copolymers. Anal Chim Acta 538:85
Liua Y, Changa X, Hua X, Guob Y, Mengb S, Wang F (2005) Highly selective determination of total mercury(II) sub microgram per liter by cyclodextrin polymer solid phase spectrophotometry using 1,3-di-(4-nitrodiazoamino)-benzene. Anal Chim Acta 532:121
Lopez-Torres E, Mendiola MA, Pastor CJ (2006) Mercury and methylmercury complexes with a triazine-3-thione ligand. Polyhedron 25:1464
Martin-Doimeadios RRC, Krupp E, Amouroux D, Donard OFX (2002) Application of isotopically labeled methylmercury for isotope dilution analysis of biological samples using gas chromatography/ICPMS. Anal Chem 74:2505
Martinis EM, Berton P, Olsina RA, Altamirano JC, Wuilloud RG (2009) Trace mercury determination in drinking and natural water samples by room temperature ionic liquid based-preconcentration and flow injection-cold vapor atomic absorption spectrometry. J Hazard Mater 167:475
Meera R, Francis T, Reddy MLP (2001) Studies on the liquid-liquid extraction of mercury(II) from acidic chloride solutions using cyanex 923. Hydrometallurgy 61:97
Naika RM, Agarwala A, Prasad S (2009) Determination of trace amounts of mercury(II) in water samples using a novel kinetic catalytic ligand substitution reaction of hexacyanoruthenate(II). Spectrochim Acta A 74:887
Nambiar DC, Patil NN, Shinde VM (1998) Liquid-liquid extraction of mercury (II) with triphenylphosphine sulphide: application to medicinal and environmental samples. Fresenius' J Anal Chem 360:205
Osawa T, Hatsukawa Y, Appel PWU, Matsue H (2011) Mercury and gold concentrations of highly polluted environmental samples determined using prompt gamma-ray analysis and instrument neutron activation analysis. Nucl Instrum Meth B 269:717
Otero-Romani J, Moreda-Pineiro A, Bermejo-Barrera A, Bermejo-Barrera P (2005) Evaluation of commercial C18 cartridges for trace elements solid phase extraction from seawater followed by inductively coupled plasma-optical emission spectrometry determination. Anal Chim Acta 536:213
Park SM, Choi HS (2002) Sensitized spectrophotometric determination of trace Hg(II) in benzalkonium chloride media. Anal Chim Acta 459:75
Rofouei MK, Rezaei A, Masteri-Farahani M, Khani H (2012) Selective extraction and preconcentration of ultra-trace level of mercury ions in water and fish samples using Fe3O4-magnetite-nanoparticles functionalized by triazene compound prior to its determination by inductively coupled plasma-optical emission spectrometry. Anal Method 4:959
Roy K, Lahiri S (2009) Extraction of Hg(I), Hg(II) and methylmercury using polyethylene glycol based aqueous biphasic system. Appl Radiat Isotopes 67:1781
Saber-Tehrani M, Givianrad MH, Hashemi-Moghaddam H (2007) Determination of total and methyl mercury in human permanent healthy teeth by electrothermal atomic absorption spectrometry after extraction in organic phase. Talanta 71:1319
Sato T, Enokida T, Noguchi Y (2002) Liquid-liquid extraction of mercury(II) from hydrochloric acid solutions by tributyl phosphate. Solvent Extr Res Dev 9:1
Segade SR, Tyson JF (2007) Determination of methylmercury and inorganic mercury in water samples by slurry sampling cold vapor atomic absorption spectrometry in a flow injection system after preconcentration on silica C18 modified. Talanta 71:1696
Shamsipur M, Ghiasvand A, Sharghi H (2002) Preconcentration of ultra trace Hg(II) in aqueous samples on octadecyl silica membrane disks modified by dibenzodiazathia-18-crown-6-dione and its determination by cold vapor atomic absorption spectrometry. Int J Environ Anal Chem 82:23
Singh DK, Mishra S (2010) Synthesis and characterization of Hg(II)-ion-imprinted polymer: kinetic and isotherm studies. Desalination 257:177
Sohrabi MR, Farokhi E, Adnani A, Ziaian M (2007) Determination of trace mercury by cloud point extraction preconcentration coupled with spectrophotometry. J Appl Sci 7:3123
Soleimani M, Mahmodi MS, Morsali A, Khani A, Ghahraman Afshar M (2011) Using a new ligand for solid phase extraction of mercury. J Hazard Mater 189:371
Soliman EM, Saleh MB, Ahmed SA (2004) New solid phase extractors for selective separation and preconcentration of mercury (II) based on silica gel immobilized aliphatic amines 2-thiophenecarboxaldehyde Schiff’s bases. Anal Chim Acta 523:133
Starvin AM, Rao TP (2004) Removal and recovery of mercury(II) from hazardous wastes using 1-(2-thiazolylazo)-2-naphthol functionalized activated carbon as solid phase extractant. J Hazard Mater 113:75
Tuzen M, Saygi KO, Soylak M (2008) Novel solid phase extraction procedure for gold(III) on Dowex M 4195 prior to its flame atomic absorption spectrometric determination. J Hazard Mater 156:591
Tuzen M, Karaman I, Citak D, Soylak M (2009) Mercury(II) and methyl mercury determinations in water and fish samples by using solid phase extraction and cold vapour atomic absorption spectrometry combination. Food Chem Toxicol 47:1648
Vereda Alonso E, Siles Cordero MT, Garca de Torres A, Canada Rudner P, Cano Pavn JM (2008) Mercury speciation in sea food by flow injection cold vapor atomic absorption spectrometry using selective solid phase extraction. Talanta 77:53
Wang L, Hu Q, Guangyu Y, Yin J, Yuan Z (2003) Determination of lead, cadmium, and mercury by on-line enrichment followed by RP-HPLC. J Anal Chem 58:1054
Wuilloud JCA, Wuilloud RG, Silva MF, Olsina RA, Martinez LD (2002) Sensitive determination of mercury in tap water by cloud point extraction pre-concentration and flow injection-cold vapor-inductively coupled plasma optical emission spectrometry. Spectrochim Acta B 57:365
Yantasee A, Warner CL, Sangvnich T, Addleman RS, Carter TG, Wiacek RJ, Fryxell GE, Stimchalk C, Warner MG (2007) Removal of heavy metals from aqueous systems with thiol functionalized super paramagnetic nanoparticles. Environ Sci Technol 41:5114
Yin C, Iqbal J, Hu H, Liu B, Zhang L, Zhu B, Du Y (2012) Sensitive determination of trace mercury by UV-visible diffuse reflectance spectroscopy after complexation and membrane filtration-enrichment. J Hazard Mater 233–234:207
Zhao XL, Cai YQ, Wang T, Shi YL, Jiang GB (2008) Preparation of alkanethiolate-functionalized core/shell Fe3O4@Au nanoparticles and its interaction with several typical target molecules. Anal Chem 80:9091
Conflict of Interest
Homeira Ebrahimzadeh has received research grants from Shahid Beheshti University. Laleh Adlnasab declares that she has no conflict of interest. Ali Akbar Asgharinezhad declares that he has no conflict of interest. Mahnaz Nasiri Aghdam declares that she has no conflict of interest. Ali Dehghani declares that he has no conflict of interest. Sousan Esmaeilpour declare that she has no conflict of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adlnasab, L., Ebrahimzadeh, H., Asgharinezhad, A.A. et al. A Preconcentration Procedure for Determination of Ultra-Trace Mercury (II) in Environmental Samples Employing Continuous-Flow Cold Vapor Atomic Absorption Spectrometry. Food Anal. Methods 7, 616–628 (2014). https://doi.org/10.1007/s12161-013-9664-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-013-9664-y