Abstract
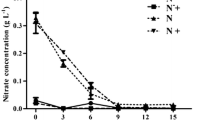
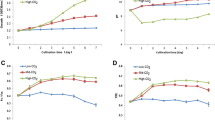
Energy crises and climate change attracted less-explored microalgae as renewable resources. Deficiencies of nitrogen and phosphorus are the most effective inducers of lipid accumulation in microalgae but at the cost of biomass productivity. Therefore, nitrogen, phosphorus, and carbon manipulation of the culture medium was adopted for maximizing lipid as well as biomass production in Dunaliella salina. Phosphate deficiency in combination with 1.25 mM KNO3 (1/8 of the basal) resulted in higher lipid content (341.1 mg g−1 dry cell weight, DCW), but lower biomass (13.12 mgL−1d−1 DCW). The addition of 10.00 mM NaHCO3 to such cultures enhanced not only lipid content to 1.17-fold but also biomass productivity to 2.25-fold. The increase in biomass may be correlated with the stress-ameliorating effects of bicarbonate augmentation which helped in maintaining the health of the cells, as reflected by robust photosynthetic performance. The two important enzymes, RuBisCO and ACCase were also monitored for their expressions. RuBisCO possesses large and small subunits (rbcL and rbcS) responsible for incorporation of CO2, and beta carboxyl transferase (accD) of the heteromeric ACCase is associated with the first and committed step of fatty acid biosynthesis. Enhanced biomass and lipid content in D. salina cells after NaHCO3 augmentation may be ascribed to 6.23-fold increase in the expression of accD and > 2.16-fold increase in rbcL and rbcS genes. Thus, the present work recommends a threshold level of nitrogen and bicarbonate in phosphate deficient D. salina cultures for simultaneously maximizing the biomass and lipid content.






Similar content being viewed by others
References
Field JL, Richard TL, Smithwick EA, Cai H, Laser MS, LeBauer DS, Long SP, Paustian K, Qin Z, Sheehan JJ, Smith P (2020) Robust paths to net greenhouse gas mitigation and negative emissions via advanced biofuels. Proc Natl Acad Sci 117(36):21968–21977. https://doi.org/10.1073/pnas.1920877117
Urtubia HO, Betanzo LB, Vásquez M (2016) Microalgae and cyanobacteria as green molecular factories: tools and perspectives. In: Thajuddin N, Dhanasekaran D (ed) Algae—Organisms for Imminent Biotechnology June 2016, Intech Open, London, pp-1–27
Chisti Y (2008) Biodiesel from microalgae beats bioethanol. Trends Biotechnol 26(3):126–131. https://doi.org/10.1016/j.tibtech.2007.12.002
Pirwitz K, Rihko-Struckmann L, Sundmacher K (2015) Comparison of flocculation methods for harvesting Dunaliella. Bioresour Technol 196:145–152. https://doi.org/10.1016/j.biortech.2015.07.032
Cardoso LG, Duarte JH, Costa JAV, de Jesus Assis D, Lemos PVF, Druzian JI, ... Chinalia FA (2021) Spirulina sp. as a bioremediation agent for aquaculture wastewater: production of high added value compounds and estimation of theoretical biodiesel. Bioenergy Res 14(1): 254–264. https://doi.org/10.1007/s12155-020-10153-4
Wilson MH, Shea A, Groppo J, Crofcheck C, Quiroz D, Quinn JC, Crocker M (2021) Algae-based beneficial re-use of carbon emissions using a novel photobioreactor: a techno-economic and life cycle analysis. Bioenergy Res 14(1):292–302. https://doi.org/10.1007/s12155-020-10178-9
Khadim SR, Singh P, Singh AK, Tiwari A, Mohanta A, Asthana RK (2018) Mass cultivation of Dunaliella salina in a flat plate photobioreactor and its effective harvesting. Bioresour Technol 270:20–29. https://doi.org/10.1016/j.biortech.2018.08.071
Ahmed RA, He M, Aftab RA, Zheng S, Nagi M, Bakri R, Wang C (2017) Bioenergy application of Dunaliella salina SA 134 grown at various salinity levels for lipid production. Sci Rep 7(1):1–10. https://doi.org/10.1038/s41598-017-07540-x
Bonnefond H, Moelants N, Talec A, Bernard O, Sciandra A (2016) Concomitant effects of light and temperature diel variations on the growth rate and lipid production of Dunaliella salina. Algal Res 14:72–78. https://doi.org/10.1016/j.algal.2015.12.018
Byrd SM, Burkholder JM (2017) Environmental stressors and lipid production in Dunaliella spp. II. Nutrients, pH, and light under optimal or low salinity. J Exp Mar Biol Ecol 487:33–44. https://doi.org/10.1016/j.jembe.2016.11.006
Singh P, Guldhe A, Kumari S, Rawat I, Bux F (2015) Investigation of combined effect of nitrogen, phosphorus and iron on lipid productivity of microalgae Ankistrodesmus falcatus KJ671624 using response surface methodology. Biochem Eng J 94:22–29. https://doi.org/10.1016/j.bej.2014.10.019
Gardner RD, Cooksey KE, Mus F, Macur R, Moll K, Eustance E, Carlson RP, Gerlach R, Fields MW, Peyton BM (2012) Use of sodium bicarbonate to stimulate triacylglycerol accumulation in the chlorophyte Scenedesmus sp. and the diatom Phaeodactylum tricornutum. J Appl Phycol 24(5):1311–1320. https://doi.org/10.1007/s10811-011-9782-0
Srinivasan R, Mageswari A, Subramanian P, Suganthi C, Chaitanyakumar A, Aswini V, Gothandam KM (2018) Bicarbonate supplementation enhances growth and biochemical composition of Dunaliella salina V-101 by reducing oxidative stress induced during macronutrient deficit conditions. Sci Rep 8(1):1–14. https://doi.org/10.1038/s41598-018-25417-5
Behera B, Unpaprom Y, Ramara R, Maniam GP, Govindan N, Paramasivan B (2021) Integrated biomolecular and bioprocess engineering strategies for enhancing the lipid yield from microalgae. Renew Sust Ener Rev 148:111270. https://doi.org/10.1016/j.rser.2021.111270
Sharma KK, Schuhmann H, Schenk PM (2012) High lipid induction in microalgae for biodiesel production. Energy J 5(5):1532–1553. https://doi.org/10.3390/en5051532
Singh P, Kumari S, Guldhe A, Singh G, Bux F (2017) ACCase and rbcL gene expression as a function of nutrient and metal stress for enhancing lipid productivity in Chlorella sorokiniana. Energy Convers Manag 148:809–819. https://doi.org/10.1016/j.enconman.2017.06.054
Cao J, Yuan H, Li B, Yang J (2014) Significance evaluation of the effects of environmental factors on the lipid accumulation of Chlorella minutissima UTEX 2341 under low-nutrition heterotrophic condition. Bioresour Technol 152:177–184. https://doi.org/10.1016/j.biortech.2013.10.084
Mirizadeh S, Nosrati M, Shojaosadati SA (2020) Synergistic effect of nutrient and salt stress on lipid productivity of Chlorella vulgaris through two-stage cultivation. Bioenergy Res 13(2):507–517. https://doi.org/10.1007/s12155-019-10077-8
Goyal A, Shiraiwa Y, Husic HD, Tolbert NE (1992) External and internal carbonic anhydrases in Dunaliella species. Mar Biol 113(3):349–355. https://doi.org/10.1007/BF00349158
Singh P, Khadim R, Singh AK, Singh U, Maurya P, Tiwari A, Asthana RK (2019) Biochemical and physiological characterization of a halotolerant Dunaliella salina isolated from hypersaline Sambhar Lake. India J Phycol 55(1):60–73. https://doi.org/10.1111/jpy.12777
Sasaki Y, Nagano Y (2004) Plant acetyl-CoA carboxylase: structure, biosynthesis, regulation, and gene manipulation for plant breeding. Biosci Biotechnol Biochem 68(6):1175–1184. https://doi.org/10.1271/bbb.68.1175
Madoka Y, Tomizawa KI, Mizoi J, Nishida I, Nagano Y, Sasaki Y (2002) Chloroplast transformation with modified accD operon increases acetyl-CoA carboxylase and causes extension of leaf longevity and increase in seed yield in tobacco. Plant Cell Physiol 43(12):1518–1525. https://doi.org/10.1093/pcp/pcf172
Andersson I, Backlund A (2008) Structure and function of Rubisco Plant Physiol. Biochem 46(3):275–291. https://doi.org/10.1016/j.plaphy.2008.01.001
Gao Y, Yang M, Wang C (2013) Nutrient deprivation enhances lipid content in marine microalgae. Bioresour Technol 147:484–491. https://doi.org/10.1016/j.biortech.2013.08.066
Bongiovani N, Popovich CA, Martínez AM, Constenla D, Leonardi PI (2020) Biorefinery approach from Nannochloropsis oceanica CCALA 978: neutral lipid and carotenoid co-production under nitrate or phosphate deprivation. Bioenergy Res 13(2):518–529. https://doi.org/10.1007/s12155-019-10045-2
Yen HW, Hu IC, Chen CY, Ho SH, Lee DJ, Chang JS (2013) Microalgae-based biorefinery–from biofuels to natural products. Bioresour Technol 135:166–174. https://doi.org/10.1016/j.biortech.2012.10.099
Minhas AK, Hodgson P, Barrow CJ, Adholeya A (2016) A review on the assessment of stress conditions for simultaneous production of microalgal lipids and carotenoids. Front Microbiol 7:546. https://doi.org/10.3389/fmicb.2016.00546
Borovkov AB, Gudvilovich IN, Avsiyan AL (2020) Scale-up of Dunaliella salina cultivation: from strain selection to open ponds. J Appl Phycol 32(3):1545–1558. https://doi.org/10.1007/s10811-020-02104-5
Chen Y, Tang X, Kapoore RV, Xu C, Vaidyanathan S (2015) Influence of nutrient status on the accumulation of biomass and lipid in Nannochloropsis salina and Dunaliella salina. Energy Convers Manag 106:61–72. https://doi.org/10.1016/j.enconman.2015.09.025
Wellburn AR, Lichtenthaler H (1984) Formulae and program to determine total carotenoids and chlorophylls A and B of leaf extracts in different solvents. In: Sybesma C (ed) Advances in photosynthesis research. Advances in Agricultural Biotechnology, vol 2. Springer, Dordrecht, pp 9–12. https://doi.org/10.1007/978-94-017-6368-4_3
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917. https://doi.org/10.1139/o59-099
Yilancioglu K, Cokol M, Pastirmaci I, Erman B, Cetiner S (2014) Oxidative stress is a mediator for increased lipid accumulation in a newly isolated Dunaliella salina strain. PLoS ONE 9(3):e91957. https://doi.org/10.1371/journal.pone.0091957
Hathwaik LT, Redelman D, Samburova V, Zielinska B, Shintani DK, Harper JF, Cushman JC (2015) Transgressive, reiterative selection by continuous buoyant density gradient centrifugation of Dunaliella salina results in enhanced lipid and starch content. Algal Res 9:194–203. https://doi.org/10.1016/j.algal.2015.03.009
Murchie EH, Lawson T (2013) Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Exp Bot 64(13):3983–3998. https://doi.org/10.1093/jxb/ert208
Tiwari A, Singh P, Riyazat Khadim S, Singh AK, Singh U, Singh P, Asthana RK (2019) Role of Ca2+ as protectant under heat stress by regulation of photosynthesis and membrane saturation in Anabaena PCC 7120. Protoplasma 256(3):681–691. https://doi.org/10.1007/s00709-018-1328-8
Hong L, Liu JL, Midoun SZ, Miller PC (2017) Transcriptome sequencing and annotation of the halophytic microalga Dunaliella salina. J Zhejiang Univ Sci B 18(10):833–844. https://doi.org/10.1631/jzus.B1700088.10.1631/jzus.B1700088
Bental M, Pick U, Avron M, Degani H (1991) Polyphosphate metabolism in the alga Dunaliella salina studied by 31P-NMR. Biochim Biophys Acta Mol Cell Res 1092(1):21–28. https://doi.org/10.1016/0167-4889(91)90173-U
Fu L, Li Q, Yan G, Zhou D, Crittenden JC (2019) Hormesis effects of phosphorus on the viability of Chlorella regularis cells under nitrogen limitation. Biotechnol Biofuels 12(1):1–9. https://doi.org/10.1186/s13068-019-1458-z
Uarrota VG, Stefen DLV, Leolato LS, Gindri DM, Nerling D (2018) Revisiting carotenoids and their role in plant stress responses: from biosynthesis to plant signalling mechanisms during stress. In: Gupta D, Palma J, Corpas F (eds) Antioxidants and antioxidant enzymes in higher plants. Springer, Cham. https://doi.org/10.1007/978-3-319-75088-0_10
Cui M, Liu Y, Zhang J (2020) The variation of growth rate, photosynthetic activity, and biodiesel productivity in Synechocystis sp. PCC 6803 under antibiotic exposure. Bioenergy Res 13(3):955–962. https://doi.org/10.1007/s12155-020-10114-x
Brindley Alías C, García-Malea López MC, Acién Fernández FG, Ferníndez Sevilla JM, García Sánchez JL, Molina Grima E (2004) Influence of power supply in the feasibility of Phaeodactylum tricornutum cultures. Biotechnol Bioeng 87(6):723–733. https://doi.org/10.1002/bit.20179
Bemejo-Padilla E, Kinsou H, Filali R, Perez-Bibbins B, Taidi B (2021) Rapid indicators for monitoring the health of Chlamydomonas nivalis biomass during preservation. J Appl Phycol 33(5):2723–2732. https://doi.org/10.1007/s10811-021-02517-w
Burrows EH, Bennette NB, Carrieri D, Dixon JL, Brinker A, Frada M, Baldassano SN, Falkowski PG, Dismukes GC (2012) Dynamics of lipid biosynthesis and redistribution in the marine diatom Phaeodactylum tricornutum under nitrate deprivation. Bioenergy Res 5(4):876–885. https://doi.org/10.1007/s12155-012-9201-7
Liang K, Zhang Q, Gu M, Cong W (2013) Effect of phosphorus on lipid accumulation in freshwater microalga Chlorella sp. J Appl Phycol 25(1):311–318. https://doi.org/10.1007/s10811-012-9865-6
Burch AR, Franz AK (2016) Combined nitrogen limitation and hydrogen peroxide treatment enhances neutral lipid accumulation in the marine diatom Phaeodactylum tricornutum. Bioresour Technol 219:559–565. https://doi.org/10.1016/j.biortech.2016.08.010
Almutairi AW (2020) Effects of nitrogen and phosphorus limitations on fatty acid methyl esters and fuel properties of Dunaliella salina. Environ Sci Pollut Res 27(26):32296–32303. https://doi.org/10.1007/s11356-020-08531-8
Burns BD, Beardall J (1987) Utilization of inorganic carbon by marine microalgae. J Exp Mar Biol Ecol 107(1):75–86. https://doi.org/10.1016/0022-0981(87)90125-0
Moll KM, Gardner RD, Eustance EO, Gerlach R, Peyton BM (2014) Combining multiple nutrient stresses and bicarbonate addition to promote lipid accumulation in the diatom RGd-1. Algal Res 5:7–15. https://doi.org/10.1016/j.algal.2014.04.002
Srinivasan R, Kumar VA, Kumar D, Ramesh N, Babu S, Gothandam KM (2015) Effect of dissolved inorganic carbon on β-carotene and fatty acid production in Dunaliella sp.. Appl Biochem Biotechnol 175(6):2895–906. https://doi.org/10.1007/s12010-014-1461-6
White DA, Pagarette A, Rooks P, Ali ST (2013) The effect of sodium bicarbonate supplementation on growth and biochemical composition of marine microalgae cultures. J Appl Phycol 25(1):153–165. https://doi.org/10.1007/s10811-012-9849-6
Qi M, Yao C, Sun B, Cao X, Fei Q, Liang B, Ran W, Xiang Q, Zhang Y, Lan X (2019) Application of an in situ CO2–bicarbonate system under nitrogen depletion to improve photosynthetic biomass and starch production and regulate amylose accumulation in a marine green microalga Tetraselmis subcordiformis. Biotechnol Biofuels 12(1):1–21. https://doi.org/10.1186/s13068-019-1523-7
Pancha I, Chokshi K, Maurya R, Trivedi K, Patidar SK, Ghosh A, Mishra S (2015) Salinity induced oxidative stress enhanced biofuel production potential of microalgae Scenedesmus sp. CCNM 1077. Bioresour Technol 189:341–348. https://doi.org/10.1016/j.biortech.2015.04.017
Shevela D, Eaton-Rye JJ, Shen JR (2012) Photosystem II and the unique role of bicarbonate: a historical perspective. Biochim Biophys Acta (BBA)-Bioenergetics 1817(8):1134–1151. https://doi.org/10.1016/j.bbabio.2012.04.003
Chavoshi ZZ, Shariati M (2019) Lipid production in Dunaliella bardawil under autotrophic, heterotrophic and mixotrophic conditions. Braz J Oceanogr 67. https://doi.org/10.1590/S1679-87592019024906709
Guihéneuf F, Stengel DB (2013) LC-PUFA-enriched oil production by microalgae: accumulation of lipid and triacylglycerols containing n-3 LC-PUFA is triggered by nitrogen limitation and inorganic carbon availability in the marine haptophyte Pavlova lutheri. Mar Drugs 11(11):4246–4266. https://doi.org/10.3390/md11114246
Mondal M, Ghosh A, Oinam G, Tiwari ON, Gayen K, Halder GN (2017) Biochemical responses to bicarbonate supplementation on biomass and lipid productivity of Chlorella sp. BTA9031 isolated from coalmine area. Environ Prog Sustain Energy 36(5):1498–1506. https://doi.org/10.1002/ep.12594
Fawzy MA, Abdel-Wahab DA, Hifney AF (2017) Physiological and biochemical responses of the green alga Pachycladella chodatii (SAG 2087) to sodicity stress. Egypt J Basic Appl Sci 4(1):30–36. https://doi.org/10.1016/j.ejbas.2016.11.001
Gordillo FJ, Jiménez C, Figueroa FL, Niell FX (1998) Effects of increased atmospheric CO2 and N supply on photosynthesis, growth and cell composition of the cyanobacterium Spirulina platensis (Arthrospira). J Appl Phycol 10(5):461–469. https://doi.org/10.1023/A:1008090402847
Talebi AF, Tohidfar M, Bagheri A, Lyon SR, Salehi-Ashtiani K, Tabatabaei M (2014) Manipulation of carbon flux into fatty acid biosynthesis pathway in Dunaliella salina using AccD and ME genes to enhance lipid content and to improve produced biodiesel quality. Biofuel Res J 1(3):91–97. https://doi.org/10.18331/BRJ2015.1.3.6
Li YX, Zhao FJ, Yu DD (2015) Effect of nitrogen limitation on cell growth, lipid accumulation and gene expression in Chlorella sorokiniana. Braz Arch Biol Technol 58(3):462–467. https://doi.org/10.1590/S1516-8913201500391
Msanne J, Xu D, Konda AR, Casas-Mollano JA, Awada T, Cahoon EB, Cerutti H (2012) Metabolic and gene expression changes triggered by nitrogen deprivation in the photoautotrophically grown microalgae Chlamydomonas reinhardtii and Coccomyxa sp. C-169. Phytochemistry 75:50–59. https://doi.org/10.1016/j.phytochem.2011.12.007
Fan J, Cui Y, Wan M, Wang W, Li Y (2014) Lipid accumulation and biosynthesis genes response of the oleaginous Chlorella pyrenoidosa under three nutrition stressors. Biotechnol Biofuels 7(1):1–4. https://doi.org/10.1186/1754-6834-7-17
Acknowledgements
We gratefully acknowledge the Head and Coordinators of CAS in Botany, DST-FIST, ISLS, BHU for providing research facilities.
Funding
The financial supports are also acknowledged by RKA (IOE Scheme No 6031), SRK (09/013/(0574)/2015-EMR-I), AM (09/013(0733)/2017-EMR-I) to CSIR, New Delhi; PS (DSKPDF; No. F.4–2/2006(BSR)/BL/19–20/162) to UGC, PM (19/06/2016(i)EU-V).
Author information
Authors and Affiliations
Contributions
SRK: Design of the experiments, execution, data collection and ms writing; AM: Methodology characterization and experimentation; PS: Isolation of D. salina from natural sample, supply of related gene data for primer designing after transcriptomic analysis, Software, data interpretation; PM: Data analysis/interpretation and support in mass cultivation during experimentation; AKS1: Coordination in doing fluorescence microscopy and discussion, software; AKS2: Visualization, Discussion and designing help in validation; RKA: Sample collection from hypersaline Sambhar lake Rajsthan, India. Conceptualization and the direction in designing, writing of ms and compilation. All authors approve the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Riyazat Khadim, S., Mohanta, A., Singh, P. et al. A Study on Dunaliella salina Under Selected Nutrient Manipulation with Reference to the Biomass, Lipid Content Along with Expression of ACCase and RuBisCO Genes. Bioenerg. Res. 16, 622–637 (2023). https://doi.org/10.1007/s12155-022-10460-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-022-10460-y