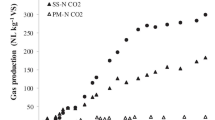
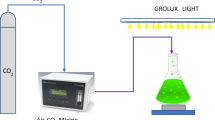
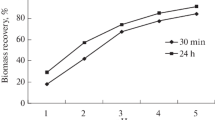
Abstract
Low-lipid microalgae such as Galdieria sulphuraria can survive extreme conditions suggesting low cultivation costs and potential industrial uses. However, so far, its energy potential for syngas and bio-oil production by pyrolysis and gasification is not fully explored. Herein, pyrolysis/gasification of Galdieria sulphuraria was studied by thermogravimetry and fixed bed reactor in a nitrogen atmosphere with/without downstream Co-Mo-based sour shift catalyst. The yield and higher heating value (HHV) of the product for each experimental run are determined and evaluated in terms of bio-char and bio-oil elemental analysis and syngas composition. Temperature greatly affects the product yield, conversion rate, and gas composition for pyrolysis experiments. However, even at high temperatures, the hydrogen content of the produced syngas is low. Low-temperature catalytic gasification experiments of Galdieria sulphuraria (500 °C) lead to the production of hydrogen-enriched syngas (41.7 vol% H2) and high HHV (~ 30 MJ/kg) bio-oil with lower oxygen and nitrogen content. The results found in this work show the potential of Galdieria sulphuraria as a renewable energy resource for high-quality oil and syngas production.





Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Florides GA, Christodoulides P (2009) Global warming and carbon dioxide through sciences. Environ Int 35(2):390–401. https://doi.org/10.1016/j.envint.2008.07.007
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev 14(2):557–577. https://doi.org/10.1016/j.rser.2009.10.009
Kumar A, Ergas S, Yuan X, Sahu A, Zhang Q, Dewulf J, Malcata FX, van Langenhove H (2010) Enhanced CO2 fixation and biofuel production via microalgae: recent developments and future directions. Trends Biotechnol 28(7):371–380. https://doi.org/10.1016/j.tibtech.2010.04.004
Barbier G, Oesterhelt C, Larson MD, Halgren RG, Wilkerson C, Garavito RM, Benning C, Weber AP (2005) Comparative genomics of two closely related unicellular thermo-acidophilic red algae, Galdieria sulphuraria and Cyanidioschyzon merolae, reveals the molecular basis of the metabolic flexibility of Galdieria sulphuraria and significant differences in carbohydrate metabolism of both algae. Plant Physiol 137(2):460–474. https://doi.org/10.1104/pp.104.051169
Castenholz RW, McDermott TR (2010) The Cyanidiales: ecology, biodiversity, and biogeography. In: Seckbach J, Chapman DJ (eds) Red algae in the genomic age. Springer Netherlands, Dordrecht, pp 357–371. https://doi.org/10.1007/978-90-481-3795-4_19
Weber A, Oesterhelt C, Gross W, Bräutigam A, Imboden L, Krassovskaya I, Linka N, Truchina J, Schneidereit J, Voll H, Voll L, Zimmermann M, Jamai A, Riekhof W, Yu B, Garavito R, Benning C (2004) EST-analysis of the thermo-acidophilic red microalga Galdieriasulphuraria reveals potential for lipid A biosynthesis and unveils the pathway of carbon export from rhodoplasts. Plant Mol Biol 55(1):17–32. https://doi.org/10.1007/s11103-004-0376-y
Hirooka S, Miyagishima S-y (2016) Cultivation of acidophilic algae Galdieria sulphuraria and Pseudochlorella sp. YKT1 in media derived from acidic hot springs. Front microbiol 7 (2022). https://doi.org/10.3389/fmicb.2016.02022
Čížková M, Vítová M, Zachleder V (2019) The red microalga Galdieria as a promising organism for applications in biotechnology. In: Vítová M (ed) Microalgae - from physiology to application. IntechOpen. https://doi.org/10.5772/intechopen.89810
Selvaratnam T, Pegallapati AK, Montelya F, Rodriguez G, Nirmalakhandan N, Van Voorhies W, Lammers PJ (2014) Evaluation of a thermo-tolerant acidophilic alga, Galdieria sulphuraria, for nutrient removal from urban wastewaters. Bioresour Technol 156:395–399. https://doi.org/10.1016/j.biortech.2014.01.075
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25(3):294–306. https://doi.org/10.1016/j.biotechadv.2007.02.001
Toor SS, Reddy H, Deng S, Hoffmann J, Spangsmark D, Madsen LB, Holm-Nielsen JB, Rosendahl LA (2013) Hydrothermal liquefaction of Spirulina and Nannochloropsis salina under subcritical and supercritical water conditions. Bioresour Technol 131:413–419. https://doi.org/10.1016/j.biortech.2012.12.144
Cheng F, Cui Z, Mallick K, Nirmalakhandan N, Brewer CE (2018) Hydrothermal liquefaction of high- and low-lipid algae: mass and energy balances. Bioresour Technol 258:158–167. https://doi.org/10.1016/j.biortech.2018.02.100
Ibrahim AFM, Dandamudi KPR, Deng S, Lin JYS (2020) Pyrolysis of hydrothermal liquefaction algal biochar for hydrogen production in a membrane reactor. Fuel 265:116935. https://doi.org/10.1016/j.fuel.2019.116935
Pan P, Hu C, Yang W, Li Y, Dong L, Zhu L, Tong D, Qing R, Fan Y (2010) The direct pyrolysis and catalytic pyrolysis of Nannochloropsis sp. residue for renewable bio-oils. Bioresour Technol 101 (12):4593–4599. https://doi.org/10.1016/j.biortech.2010.01.070
Gong X, Zhang B, Zhang Y, Huang Y, Xu M (2014) Investigation on pyrolysis of low lipid microalgae Chlorella vulgaris and Dunaliella salina. Energy Fuels 28(1):95–103. https://doi.org/10.1021/ef401500z
Peng W, Wu Q, Tu P (2000) Effects of temperature and holding time on production of renewable fuels from pyrolysis of Chlorella protothecoides. J Appl Phycol 12(2):147–152. https://doi.org/10.1023/A:1008115025002
Aysu T, Sanna A (2015) Nannochloropsis algae pyrolysis with ceria-based catalysts for production of high-quality bio-oils. Bioresour Technol 194:108–116. https://doi.org/10.1016/j.biortech.2015.07.027
Thangalazhy-Gopakumar S, Adhikari S, Chattanathan SA, Gupta RB (2012) Catalytic pyrolysis of green algae for hydrocarbon production using H+ZSM-5 catalyst. Bioresour Technol 118:150–157. https://doi.org/10.1016/j.biortech.2012.05.080
Raheem A, Dupont V, Channa AQ, Zhao X, Vuppaladadiyam AK, Taufiq-Yap Y-H, Zhao M, Harun R (2017) Parametric characterization of air gasification of Chlorella vulgaris biomass. Energy Fuels 31(3):2959–2969. https://doi.org/10.1021/acs.energyfuels.6b03468
Hirano A, Hon-Nami K, Kunito S, Hada M, Ogushi Y (1998) Temperature effect on continuous gasification of microalgal biomass: theoretical yield of methanol production and its energy balance. Catal Today 45(1):399–404. https://doi.org/10.1016/S0920-5861(98)00275-2
A Raheem W. A. K. G WA, Taufiq Yap YH, Danquah MK, Harun R, 2015 Optimization of the microalgae Chlorella vulgaris for syngas production using central composite design RSC Adv 5 88 71805 71815. https://doi.org/10.1039/C5RA10503J
Díaz-Rey MR, Cortés-Reyes M, Herrera C, Larrubia MA, Amadeo N, Laborde M, Alemany LJ (2015) Hydrogen-rich gas production from algae-biomass by low temperature catalytic gasification. Catal Today 257:177–184. https://doi.org/10.1016/j.cattod.2014.04.035
Duman G, Uddin MA, Yanik J (2014) Hydrogen production from algal biomass via steam gasification. Bioresour Technol 166:24–30. https://doi.org/10.1016/j.biortech.2014.04.096
Raheem A, Liu H, Ji G, Zhao M (2019) Gasification of lipid-extracted microalgae biomass promoted by waste eggshell as CaO catalyst. Algal Res 42:101601. https://doi.org/10.1016/j.algal.2019.101601
Arnold RA, Hill JM (2019) Catalysts for gasification: a review. Sustain Energy Fuels 3(3):656–672. https://doi.org/10.1039/C8SE00614H
Ren J, Cao J-P, Zhao X-Y, Yang F-L, Wei X-Y (2019) Recent advances in syngas production from biomass catalytic gasification: a critical review on reactors, catalysts, catalytic mechanisms and mathematical models. Renew Sustain Energy Rev 116:109426. https://doi.org/10.1016/j.rser.2019.109426
Tang Z, Chen W, Chen Y, Hu J, Yang H, Chen H (2021) Preparation of low-nitrogen and high-quality bio-oil from microalgae catalytic pyrolysis with zeolites and activated carbon. J Anal Appl Pyrolysis:105182. https://doi.org/10.1016/j.jaap.2021.105182
Gong Z, Fang P, Wang Z, Li Q, Li X, Meng F, Zhang H, Liu L (2020) Catalytic pyrolysis of chemical extraction residue from microalgae biomass. Renew Energy 148:712–719. https://doi.org/10.1016/j.renene.2019.10.158
Watanabe H, Li D, Nakagawa Y, Tomishige K, Watanabe MM (2015) Catalytic gasification of oil-extracted residue biomass of Botryococcus braunii. Bioresour Technol 191:452–459. https://doi.org/10.1016/j.biortech.2015.03.034
Mo L, Dai H, Feng L, Liu B, Li X, Chen Y, Khan S (2020) In-situ catalytic pyrolysis upgradation of microalgae into hydrocarbon rich bio-oil: effects of nitrogen and carbon dioxide environment. Bioresour Technol 314:123758. https://doi.org/10.1016/j.biortech.2020.123758
Liu B, Zhao L, Wu Z, Zhang J, Zong Q, Almegren H, Wei F, Zhang X, Zhao Z, Gao J, Xiao T (2019) Recent advances in industrial sulfur tolerant water gas shift catalysts for syngas hydrogen enrichment: application of lean (low) steam/gas ratio. Catalysts 9(9):772. https://doi.org/10.3390/catal9090772
Chianese S, Fail S, Binder M, Rauch R, Hofbauer H, Molino A, Blasi A, Musmarra D (2016) Experimental investigations of hydrogen production from CO catalytic conversion of tar rich syngas by biomass gasification. Catal Today 277:182–191. https://doi.org/10.1016/j.cattod.2016.04.005
de la Osa AR, De Lucas A, Valverde JL, Romero A, Monteagudo I, Sánchez P (2011) Performance of a sulfur-resistant commercial WGS catalyst employing industrial coal-derived syngas feed. Int J Hydrog Energy 36(1):44–51. https://doi.org/10.1016/j.ijhydene.2010.08.127
Ratnasamy C, Wagner JP (2009) Water gas shift catalysis. Catal Rev 51(3):325–440. https://doi.org/10.1080/01614940903048661
Dandamudi KPR, Muppaneni T, Sudasinghe N, Schaub T, Holguin FO, Lammers PJ, Deng S (2017) Co-liquefaction of mixed culture microalgal strains under sub-critical water conditions. Bioresour Technol 236:129–137. https://doi.org/10.1016/j.biortech.2017.03.165
Wang J, Krishna R, Yang J, Dandamudi KPR, Deng S (2015) Nitrogen-doped porous carbons for highly selective CO2 capture from flue gases and natural gas upgrading. Mater Today Commun 4:156–165. https://doi.org/10.1016/j.mtcomm.2015.06.009
Dandamudi KPR, Muppaneni T, Markovski JS, Lammers P, Deng S (2019) Hydrothermal liquefaction of green microalga Kirchneriella sp. under sub- and super-critical water conditions. Biomass Bioenergy 120:224–228. https://doi.org/10.1016/j.biombioe.2018.11.021
Waldheim L, Nilsson T (2001) Heating value of gases from biomass gasification. Report Number: TPS-01/16 S. TPS Termiska Processer AB, 611 82 Nyköping. From http://gasificationofbiomass.org/app/webroot/files/file/publications/HeatingValue.pdf
Wang J, Xiao B, Liu S, Hu Z, He P, Guo D, Hu M, Qi F, Luo S (2013) Catalytic steam gasification of pig compost for hydrogen-rich gas production in a fixed bed reactor. Bioresour Technol 133:127–133. https://doi.org/10.1016/j.biortech.2013.01.092
Vasudevan PT, Briggs M (2008) Biodiesel production–current state of the art and challenges. J Ind Microbiol Biotechnol 35(5):421. https://doi.org/10.1007/s10295-008-0312-2
McKendry P (2002) Energy production from biomass (part 1): overview of biomass. Bioresour Technol 83(1):37–46. https://doi.org/10.1016/S0960-8524(01)00118-3
Mohan D, Pittman CU, Steele PH (2006) Pyrolysis of wood/biomass for bio-oil: a critical review. Energy Fuels 20(3):848–889. https://doi.org/10.1021/ef0502397
Pinto F, Franco C, André RN, Tavares C, Dias M, Gulyurtlu I, Cabrita I (2003) Effect of experimental conditions on co-gasification of coal, biomass and plastics wastes with air/steam mixtures in a fluidized bed system. Fuel 82(15):1967–1976. https://doi.org/10.1016/S0016-2361(03)00160-1
Florin NH, Harris AT (2008) Enhanced hydrogen production from biomass with in situ carbon dioxide capture using calcium oxide sorbents. Chem Eng Sci 63(2):287–316. https://doi.org/10.1016/j.ces.2007.09.011
De la Osa AR, De Lucas A, Romero A, Valverde JL, Sánchez P (2011) Kinetic models discrimination for the high pressure WGS reaction over a commercial CoMo catalyst. Int J Hydrogen Energy 36(16):9673–9684. https://doi.org/10.1016/j.ijhydene.2011.05.043
Bae YJ, Ryu C, Jeon J-K, Park J, Suh DJ, Suh Y-W, Chang D, Park Y-K (2011) The characteristics of bio-oil produced from the pyrolysis of three marine macroalgae. Bioresour Technol 102(3):3512–3520. https://doi.org/10.1016/j.biortech.2010.11.023
Thangalazhy-Gopakumar S, Adhikari S, Ravindran H, Gupta RB, Fasina O, Tu M, Fernando SD (2010) Physiochemical properties of bio-oil produced at various temperatures from pine wood using an auger reactor. Bioresour Technol 101(21):8389–8395. https://doi.org/10.1016/j.biortech.2010.05.040
Li F, Srivatsa SC, Batchelor W, Bhattacharya S (2017) A study on growth and pyrolysis characteristics of microalgae using thermogravimetric analysis-infrared spectroscopy and synchrotron Fourier transform infrared spectroscopy. Bioresour Technol 229:1–10. https://doi.org/10.1016/j.biortech.2017.01.005
Li F, Srivatsa SC, Bhattacharya S (2019) A review on catalytic pyrolysis of microalgae to high-quality bio-oil with low oxygeneous and nitrogenous compounds. Renew Sustain Energy Rev 108:481–497. https://doi.org/10.1016/j.rser.2019.03.026
Nolte MW, Saraeian A, Shanks BH (2017) Hydrodeoxygenation of cellulose pyrolysis model compounds using molybdenum oxide and low pressure hydrogen. Green Chem 19(15):3654–3664. https://doi.org/10.1039/C7GC01477E
Xu L, Keogh RA, Huang C-S, Spicer RL, Sparks DE, Lambert S, Thomas GA, Davis BH (1994) Catalytic hydrotreatment of coal-derived naphtha. Fuel Sci Technol Int 12(10):1355–1376. https://doi.org/10.1080/08843759408916239
Ruddy DA, Schaidle JA, Ferrell Iii JR, Wang J, Moens L, Hensley JE (2014) Recent advances in heterogeneous catalysts for bio-oil upgrading via “ex situ catalytic fast pyrolysis”: catalyst development through the study of model compounds. Green Chem 16(2):454–490. https://doi.org/10.1039/C3GC41354C
Acknowledgements
The authors are grateful to Dr. K.P.R. Dandamudi and Prof. Peter Lammers for providing algae samples in this study.
Author information
Authors and Affiliations
Contributions
Fateme Banihashemi: conceptualization, performing experiments, and characterization work. Amr F. M. Ibrahim: conceptualization, experiment design, formal analysis, data curation, writing — original draft. Shuguang Deng: supervision, project administration, writing — review and editing. Y.S. Lin: supervision, project administration, writing — review and editing.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
There are no ethical issues associated with this manuscript and all the author consent to participate.
Consent for Publication
All authors consent the research article for publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Banihashemi, F., Ibrahim, A.F.M., Deng, S. et al. Pyrolysis and Gasification Characteristics of Galdieria sulphuraria Microalgae. Bioenerg. Res. 16, 611–621 (2023). https://doi.org/10.1007/s12155-022-10449-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-022-10449-7