Abstract
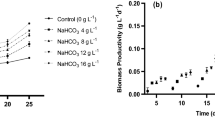
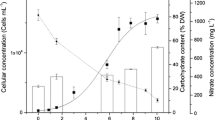
Spirulina platensis, when grown under stress, may alter its metabolic pathways, leading to carbohydrate accumulation. Research on the combined effects of stress factors such as UV radiation, photoperiod, and light, and the influence of micronutrients (Ca, Fe, and Mg) on microalgae carbohydrate composition is scarce. The aim of the present study was to evaluate the effects of combined cell stress factors on the microalgal growth and biochemical composition of S. platensis, to apply the biomass in the context of biorefineries. Assays were performed at two cultivation stages: in the first stage, Zarrouk medium (50%) was used until the end of the exponential growth phase (18 days) and, in the second stage, the cells obtained in the first stage were centrifuged and recultivated in 20% Zarrouk’s medium under physical (UV, photoperiod/light, NaCl) and nutritional stress (limitation of Ca, Fe, and Mg concentrations in 20% Zarrouk). The highest carbohydrate yields (27.84 mg L−1 day−1) were obtained in the photoperiod/light intensity-stressed cultures in the medium with nutrient limitation. The study outcomes showed the methods to increase carbohydrate synthesis in cultures under optimized conditions. Our results showed that nutrient limitation by 20% Zarrouk combined with higher light intensity (67.5 μmol photons m−2 s−1) and photoperiod (18-h/6-h light/dark cycles) is an efficient strategy to achieve higher intracellular concentrations (59.71%) and carbohydrate yields (55.85 mg L−1 day−1) in a discontinuous culture method.







Similar content being viewed by others
References
Brennan L, Owende P (2010) Biofuels from microalgae-a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energ Rev 14:557–577. https://doi.org/10.1016/j.rser.2009.10.009
Deprá MC, dos Santos AM, Severo IA, Santos AB, Zepka LQ, Jacob-Lopes E (2018) Microalgal biorefineries for bioenergy production: can we move from concept to industrial reality? Bioenergy Res 11:727–747
Fan J, Lvhong Z (2017) Acclimation to NaCl and light stress of heterotrophic Chlamydomonas reinhardtii for lipid accumulation. J Biosci Bioeng 124:302–308. https://doi.org/10.1016/j.jbiosc.2017.04.009
Shuba ES, Kifle D (2018) Microalgae to biofuels: ‘promising’ alternative and renewable energy, review. Renew Sust Energ Rev 81:743–755. https://doi.org/10.1016/j.rser.2017.08.042
Milano J, Ong HC, Masjuki HH, Chong WT, Lam MK, Loh PK, Vellayan V (2016) Microalgae biofuels as an alternative to fossil fuel for power generation. Renew Sust Energ Rev 58:180–197. https://doi.org/10.1016/j.rser.2015.12.150
Rempel A, Machado T, Treichel H, Colla E, Margarites AC, Colla LM (2018) Saccharification of Spirulina platensis biomass using free and immobilized amylolytic enzymes. Bioresour Technol 263:163–171. https://doi.org/10.1016/j.biortech.2018.04.114
Khan MI, Shin JH, Kim JD (2018) The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Factories 17:1–21
Markou G, Nerantzis E (2013) Microalgae for high-value compounds and biofuels production: a review with focus on cultivation under stress conditions. Biotechnol Adv 31:1532–1542. https://doi.org/10.1016/j.biotechadv.2013.07.011
Silva CEF, Sforza E (2016) Carbohydrate productivity in continuous reactor under nitrogen limitation: effect of light and residence time on nutrient uptake in Chlorella vulgaris. Process Biochem 51:2112–2118. https://doi.org/10.1016/j.procbio.2016.09.015
Magro FG, Margarites AC, Reinehr CO, Gonçalves GC, Rodigheri G, Costa JAV, Colla LM (2018) Spirulina platensis biomass composition is influenced by the light availability and harvest phase in raceway ponds. Environ Technol 39:1868–1877. https://doi.org/10.1080/09593330.2017.1340352
Salla ACV, Margarites AC, Seibel FI, Holz LC, Brião VB, Bertolin TE, Colla LM, Costa JAV (2016) Increase in the carbohydrate content of the microalgae Spirulina in culture by nutrient starvation and the addition of residues of whey protein concentrate. Bioresour Technol 209:133–141. https://doi.org/10.1016/j.biortech.2016.02.069
Li X, Li W, Zhai J, Wei H (2018) Effect of nitrogen limitation on biochemical composition and photosynthetic performance for fed-batch mixotrophic cultivation of microalga Spirulina platensis. Bioresour Technol 263:555–561. https://doi.org/10.1016/j.biortech.2018.05.046
Costa JAV, Freitas BCB, Rosa GM, Moraes L, Morais MG, Mitchell BG (2019) Operational and economic aspects of Spirulina-based biorefinery. Bioresour Technol 292:121946. https://doi.org/10.1016/j.biortech.2019.121946
Cheng D, He Q (2014) Assessment of environmental stresses for enhanced microalgal biofuel production - an overview. Front Energy Res 2:26. https://doi.org/10.3389/fenrg.2014.00026
Dragone G, Fernandes BD, Abreu AP, Vicente AA, Teixeira JA (2011) Nutrient limitation as a strategy for increasing starch accumulation in microalgae. Appl Energy 88:3331–3335. https://doi.org/10.1016/j.apenergy.2011.03.012
Markou G, Chatzipavlidis I, Georgakakis D (2012) Effects of phosphorus concentration and light intensity on the biomass composition of Arthrospira (Spirulina) platensis. World J Microbiol Biotechnol 28:2661–2670. https://doi.org/10.1007/s11274-012-1076-4
Chokshi K, Pancha I, Ghosh A, Mishra S (2017) Salinity induced oxidative stress alters the physiological responses and improves the biofuel potential of green microalgae Acutodesmus dimorphus. Bioresour Technol 244:1376–1383. https://doi.org/10.1016/j.biortech.2017.05.003
Liu S, Zhao Y, Liu L, Ao X, Ma L, Wu M, Ma F (2015) Improving cell growth and lipid accumulation in green microalgae Chlorella sp. via UV irradiation. Appl Biochem Biotechnol 175:3507–3518. https://doi.org/10.1007/s12010-015-1521-6
Sharma KK, Li Y, Schenk PM (2015) Rapid lipid induction in Chlorella sp. by UV-C radiation. Bioenergy Res 8:1824–1830. https://doi.org/10.1007/s12155-015-9633-y
Mandotra SK, Kumar P, Suseela MR, Nayaka S, Ramteke PW (2016) Evaluation of fatty acid profile and biodiesel properties of microalga Scenedesmus abundans under the influence of phosphorus, pH and light intensities. Bioresour Technol 201:222–229. https://doi.org/10.1016/j.biortech.2015.11.042
Sun X, Cao Y, Xu H, Liu Y, Sun J, Qiao D, Cao Y (2014) Effect of nitrogen-starvation, light intensity and iron on triacylglyceride/carbohydrate production and fatty acid profile of Neochloris oleoabundans HK-129 by a two-stage process. Bioresour Technol 155:204–212. https://doi.org/10.1016/j.biortech.2013.12.109
Khajepour F, Hosseini SA, Ghorbani Nasrabadi R, Markou G (2015) Effect of light intensity and photoperiod on growth and biochemical composition of a local isolate of Nostoc calcicola. Appl Biochem Biotechnol 176:2279–2289. https://doi.org/10.1007/s12010-015-1717-9
Wang X, Ruan Z, Sheridan P, Boileau D, Liu Y, Liao W (2015) Two-stage photoautotrophic cultivation to improve carbohydrate production in Chlamydomonas reinhardtii. Biomass Bioenergy 74:280–287. https://doi.org/10.1016/j.biombioe.2015.01.024
Aziz MMA, Kassim KA, Shokravi Z, Jakarni FM, Lieu HY, Zaini N, Tan LS, Islam S, Shokravi H (2020) Two-stage cultivation strategy for simultaneous increases in growth rate and lipid content of microalgae: a review. Renew Sust Energ Rev 119:109621. https://doi.org/10.1016/j.rser.2019.109621
Ho SH, Ye X, Hasunuma T, Chang J, Kondo A (2014) Perspectives on engineering strategies for improving biofuel production from microalgae - a critical review. Biotechnol Adv 32:1448–1459. https://doi.org/10.1016/j.biotechadv.2014.09.002
Zhu L (2015) Microalgal culture strategies for biofuel production: a review. Biofuels Bioprod Biorefin 9:801–814. https://doi.org/10.1002/bbb.1576
Ferreira AF, Ribeiro LA, Batista AP, Marques PASS, Nobre BP, Palavra AMF, Silva PP, Gouveia L, Silva C (2013) A biorefinery from Nannochloropsis sp. microalga - energy and CO2 emission and economic analyses. Bioresour Technol 138:235–244. https://doi.org/10.1016/j.biortech.2013.03.168
Nagappan S, Devendran S, Tsai PC, Dahms HU, Ponnusamy VK (2019) Potential of two-stage cultivation in microalgae biofuel production. Fuel 252:339–349. https://doi.org/10.1016/j.fuel.2019.04.138
Polat E, Yüksel E, Altınbaş M (2020) Mutual effect of sodium and magnesium on the cultivation of microalgae Auxenochlorella protothecoides. Biomass Bioenergy 132:105441. https://doi.org/10.1016/j.biombioe.2019.105441
Di Caprio F, Altimari P, Pagnanelli F (2018) Effect of Ca2+ concentration on Scenedesmus sp. growth in heterotrophic and photoautotrophic cultivation. New Biotechnol 40:228–235. https://doi.org/10.1016/j.nbt.2017.09.003
Gorain PC, Bagchi SK, Mallick N (2013) Effects of calcium, magnesium and sodium chloride in enhancing lipid accumulation in two green microalgae. Environ Technol 34:1887–1894. https://doi.org/10.1080/09593330.2013.812668
Ren HY, Liu BF, Kong F, Zhao L, Xie GJ, Ren NQ (2014) Enhanced lipid accumulation of green microalga Scenedesmus sp. by metal ions and EDTA addition. Bioresour Technol 169:763–767. https://doi.org/10.1016/j.biortech.2014.06.062
Islami HR, Assareh R (2020) Enhancement effects of ferric ion concentrations on growth and lipid characteristics of freshwater microalga Chlorococcum oleofaciens KF584224.1 for biodiesel production. Renew Energy 149:264–272. https://doi.org/10.1016/j.renene.2019.12.067
Hanifzadeh MM, Garcia EC, Viamajala S (2018) Production of lipid and carbohydrate from microalgae without compromising biomass productivities: role of Ca and Mg. Renew Energy 127:989–997. https://doi.org/10.1016/j.renene.2018.05.012
Lim DKY, Schuhmann H, Sharma K, Schenk PM (2015) Isolation of high-lipid Tetraselmis suecica strains following repeated UV-C mutagenesis, FACS, and high-throughput growth selection. Bioenergy Res 8:750–759. https://doi.org/10.1007/s12155-014-9553-2
Morais MG, Costa JAV (2007) Carbon dioxide fixation by Chlorella kessleri, C. vulgaris, Scenedesmus obliquus and Spirulina sp. cultivated in flasks and vertical tubular photobioreactors. Biotechnol Lett 29:1349–1352. https://doi.org/10.1007/s10529-007-9394-6
Zarrouk C (1966) Contribution à l’étude d’une cyanophycée: influence de divers facteurs physiques et chimiques sur la croissance et la photosynthèse de Spirulina maxima. Thesis, Université of Paris. (In French)
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Margarites AC, Volpato N, Araújo E, Cardoso LG, Bertolin TE, Colla LM, Costa JAV (2016) Spirulina platensis is more efficient than Chlorella homosphaera in carbohydrate productivity. Environ Technol 38:2209–2216. https://doi.org/10.1080/09593330.2016.1254685
Lowry OH, Rosebrough NJ, Farr AL, Randall R (1951) Protein measurement with the Folin phenol reagent. J Biol Chem:265–276
Association of Official Analytical Chemists (2000) Official Methods of Analysis of AOAC International 17th
Lourenco S de O (2006) Cultivo de microalgas marinhas : princípios e aplicações. RiMa, São Carlos. (In Portuguese)
Fernandes BD, Mota A, Ferreira A, Dragone G, Teixeira JA, Vicente AA (2014) Characterization of split cylinder airlift photobioreactors for efficient microalgae cultivation. Chem Eng Sci 117:445–454. https://doi.org/10.1016/j.ces.2014.06.043
Pires JCM, Alvim-Ferraz MCM, Martins FG, Simões M (2012) Carbon dioxide capture from flue gases using microalgae: engineering aspects and biorefinery concept. Renew Sust Energ Rev 16:3043–3053. https://doi.org/10.1016/j.rser.2012.02.055
Álvarez-Díaz PD, Ruiz J, Arbib Z, Barragán J, Garrido-Pérez MC, Perales JA (2015) Wastewater treatment and biodiesel production by Scenedesmus obliquus in a two-stage cultivation process. Bioresour Technol 181:90–96. https://doi.org/10.1016/j.biortech.2015.01.018
Chokshi K, Pancha I, Trivedi K, George B, Maurya R, Ghosh A, Mishra S (2015) Biofuel potential of the newly isolated microalgae Acutodesmus dimorphus under temperature induced oxidative stress conditions. Bioresour Technol 180:162–171. https://doi.org/10.1016/j.biortech.2014.12.102
Esakkimuthu S, Krishnamurthy V, Govindarajan R, Swaminathan K (2016) Augmentation and starvation of calcium, magnesium, phosphate on lipid production of Scenedesmus obliquus. Biomass Bioenergy 88:126–134. https://doi.org/10.1016/j.biombioe.2016.03.019
Wan M, Jin X, Xia J, Rosenberg JN, Yu G, Nie Z, Oyler GA, Betenbaugh MJ (2014) The effect of iron on growth, lipid accumulation, and gene expression profile of the freshwater microalga Chlorella sorokiniana. Appl Microbiol Biotechnol 98:9473–9481. https://doi.org/10.1007/s00253-014-6088-6
Markou G, Vandamme D, Muylaert K (2014) Microalgal and cyanobacterial cultivation: the supply of nutrients. Water Res 65:186–202. https://doi.org/10.1016/j.watres.2014.07.025
Margarites ACF, Costa JAV (2014) Increment of carbohydrate concentration of Chlorella minutissima microalgae for bioethanol production. J Eng Res Appl 4:80–86
Zhang X, Tang X, Wang M, Zhang W, Zhou B, Wang Y (2017) ROS and calcium signaling mediated pathways involved in stress responses of the marine microalgae Dunaliella salina to enhanced UV-B radiation. J Photochem Photobiol B 173:360–367. https://doi.org/10.1016/j.jphotobiol.2017.05.038
Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI (2007) Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta 1767:414–421. https://doi.org/10.1016/j.bbabio.2006.11.019
Dismukes GC, Carrieri D, Bennette N, Ananyev GM, Posewitz MC (2008) Aquatic phototrophs: efficient alternatives to land-based crops for biofuels. Curr Opin Biotechnol 19:235–240. https://doi.org/10.1016/j.copbio.2008.05.007
Pancha I, Chokshi K, Maurya R, Trivedi K, Patidar SK, Ghosh A, Mishra S (2015) Salinity induced oxidative stress enhanced biofuel production potential of microalgae Scenedesmus sp. CCNM 1077. Bioresour Technol 189:341–348. https://doi.org/10.1016/j.biortech.2015.04.017
Kováčik J, Dresler S (2018) Calcium availability but not its content modulates metal toxicity in Scenedesmus quadricauda. Ecotoxicol Environ Saf 147:664–669. https://doi.org/10.1016/j.ecoenv.2017.09.022
Sharma K, Li Y, Schenk PM (2014) UV-C-mediated lipid induction and settling, a step change towards economical microalgal biodiesel production. Green Chem 16:3539–3548. https://doi.org/10.1039/c4gc00552j
Hu Q (2004) Environmental effects on cell composition. In: Richmond A (ed) Handbook of 560 microalgal culture: biotechnology and applied phycology. Blackwell, Oxford, pp 83–93
Wahidin S, Idris A, Shaleh SRM (2013) The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresour Technol 129:7–11. https://doi.org/10.1016/j.biortech.2012.11.032
George B, Pancha I, Desai C, Chokshi K, Paliwal C, Ghosh T, Mishra S (2014) Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus - a potential strain for bio-fuel production. Bioresour Technol 171:367–374. https://doi.org/10.1016/j.biortech.2014.08.086
Braga V d S, Mastrantonio DJ d S, Costa JAV, Morais MG d (2018) Cultivation strategy to stimulate high carbohydrate content in Spirulina biomass. Bioresour Technol 269:221–226. https://doi.org/10.1016/j.biortech.2018.08.105
Del Río E, Acién FG, García-Malea MC, Rivas J, Molina-Grima E, Guerrero MG (2008) Efficiency assessment of the one-step production of astaxanthin by the microalga Haematococcus pluvialis. Biotechnol Bioeng 100:397–402. https://doi.org/10.1002/bit.21770
Markou G, Chatzipavlidis I, Georgakakis D (2012) Carbohydrates production and bio-flocculation characteristics in cultures of Arthrospira (Spirulina) platensis: improvements through phosphorus limitation process. Bioenergy Res 5:915–925. https://doi.org/10.1007/s12155-012-9205-3
Chentir I, Doumandji A, Ammar J, Zili F, Jridi M, Markou G, Ouada HB (2018) Induced change in Arthrospira sp. (Spirulina) intracellular and extracellular metabolites using multifactor stress combination approach. J Appl Phycol 30:1563–1574. https://doi.org/10.1007/s10811-017-1348-3
Iasimone F, Panico A, De Felice V, Fantasma F, Iorizzi M, Pirozzi F (2018) Effect of light intensity and nutrients supply on microalgae cultivated in urban wastewater: biomass production, lipids accumulation and settleability characteristics. J Environ Manag 223:1078–1085. https://doi.org/10.1016/j.jenvman.2018.07.024
Wen Q, Chen Z, Li P, Han Y, Feng Y, Ren N (2013) Lipid production for biofuels from effluent-based culture by heterotrophic Chlorella protothecoides. Bioenergy Res 6:877–882. https://doi.org/10.1007/s12155-013-9308-5
Ferreira AF, Ferreira A, Dias APS, Gouveia L (2020) Pyrolysis of Scenedesmus obliquus biomass following the treatment of different wastewaters. Bioenergy Res:1–11. https://doi.org/10.1007/s12155-020-10102-1
Aketo T, Hoshikawa Y, Nojima D, Yabu Y, Maeda Y, Yoshino T, Takano H, Tanaka T (2020) Selection and characterization of microalgae with potential for nutrient removal from municipal wastewater and simultaneous lipid production. J Biosci Bioeng 129:565–572. https://doi.org/10.1016/j.jbiosc.2019.12.004
Funding
The authors are pleased to acknowledge the National Council for Scientific and Technological Development (CNPq) and Coordination of Improvement of Higher Education Personnel (CAPES) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zaparoli, M., Ziemniczak, F.G., Mantovani, L. et al. Cellular Stress Conditions as a Strategy to Increase Carbohydrate Productivity in Spirulina platensis. Bioenerg. Res. 13, 1221–1234 (2020). https://doi.org/10.1007/s12155-020-10133-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-020-10133-8