Abstract
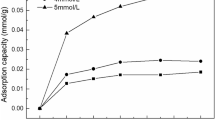
Sulfonation degree is an important structural parameter of lignosulfonates which affect their water solubility, surface activity, and dispersing performance. Presently, the methods for determining sulfonation degree include elemental analysis and potentiometric titration; they have some shortcomings such as complex purification process, high equipment requirements, and time-consuming. Hence, a simple and rapid method to determine the sulfonation degree of lignosulfonates was proposed based on the electrostatic interaction between cationic surfactant (hexadecyl trimethyl ammonium bromide (CTAB)) and anionic lignosulfonates. CTAB was added and co-precipitated with lignosulfonates and then the ultraviolet absorbance of the supernatant was determined. The sulfonation degree of lignosulfonates was calculated according to the titration curve of the supernatant absorbance to the amount of CTAB. This method is applicable to lignosulfonates recovered from acid pulping as well as sulfonation products of alkali lignin. Because of the easy operation process and no purification process for lignosulfonates, this method was simple and rapid when compared to existing methods for sulfonation degree determination of lignosulfonates.






Similar content being viewed by others
References
Aro T, Fatehi P (2017) Production and application of lignosulfonates and sulfonated lignin. Chemsuschem 10(9):1861–1877. https://doi.org/10.1002/cssc.201700082
Lou HM, Ji KB, Lin HK, Pang YX, Deng YH, Qiu XQ, Zhang HB, Xie ZG (2012) Effect of molecular weight of sulphonated acetone-formaldehyde condensate on its adsorption and dispersion properties in cementitious system. Cem Concr Res 42(8):1043–1048. https://doi.org/10.1016/j.cemconres.2011.11.002
Ouyang XP, Ke LX, Qiu XQ, Guo YX, Pang YX (2009) Sulfonation of alkali lignin and its potential use in dispersant for cement. J Dispers Sci Technol 30(1):1–6. https://doi.org/10.1080/01932690802473560
Yang DJ, Li HJ, Qin YL, Zhong RS, Bai MX, Qiu XQ (2015) Structure and properties of sodium lignosulfonate with different molecular weight used as dye dispersant. J Dispers Sci Technol 36(4):532–539. https://doi.org/10.1080/01932691.2014.916221
Mu YF, Peng YJ, Lauten RA (2018) The galvanic interaction between chalcopyrite and pyrite in the presence of lignosulfonate-based biopolymers and its effects on flotation performance. Miner Eng 122:91–98. https://doi.org/10.1016/j.mineng.2018.03.048
Ansari A, Pawlik M (2007) Floatability of chalcopyrite and molybdenite in the presence of lignosulfonates. Part I. Adsorption studies. Miner Eng 20(6):600–608. https://doi.org/10.1016/j.mineng.2006.12.007
Pang YX, Li XY, Zhou MS, Li Y, Gao W, Qiu XQ (2016) Relationship between the hydrophilicity of lignin dispersants and their performance towards pesticide particles. Holzforschung 70(7):653–660. https://doi.org/10.1515/hf-2015-0134
Pavlov D, Nikolov P, Rogachev T (2010) Influence of expander components on the processes at the negative plates of lead-acid cells on high-rate partial-state-of-charge cycling. Part I: effect of lignosulfonates and BaSO4 on the processes of charge and discharge of negative plates. J Power Sources 195(14):4435–4443. https://doi.org/10.1016/j.jpowsour.2009.12.132
Hong NL, Li Y, Zeng WM, Zhang MK, Peng XW, Qiu XQ (2015) Ultrahigh molecular weight, lignosulfonate-based polymers: preparation, self-assembly behaviours and dispersion property in coal-water slurry. RSC Adv 5(28):21588–21595. https://doi.org/10.1039/c5ra02157j
Yang D, Qiu XQ, Zhou MS, Lou HM (2007) Properties of sodium lignosulfonate as dispersant of coal water slurry. Energy Convers Manag 48(9):2433–2438. https://doi.org/10.1016/j.enconman.2007.04.007
Chandra RP, Gourlay K, Kim CS, Saddler JN (2015) Enhancing hemicellulose recovery and the enzymatic hydrolysis of cellulose by adding lignosulfonates during the two-stage steam pretreatment of poplar. ACS Sustain Chem Eng 3(5):986–991. https://doi.org/10.1021/acssuschemeng.5b00124
Li ZL, Pang YX, Ge YY, Qiu XQ (2012) Adsorption of different molecular weight lignosulfonates on dimethomorph powder in an aqueous system. J Ind Eng Chem 18(1):532–537. https://doi.org/10.1016/j.jiec.2011.11.069
Lin XL, Cai C, Huang JH, Lou HM, Qiu XQ, Lin ML, Zheng JY, Pang YX, Yang DJ (2017) Understanding the effect of the complex of lignosulfonate and cetyltrimethylammonium bromide on the enzymatic digestibility of cellulose. Energy Fuel 31(1):672–678. https://doi.org/10.1021/acs.energyfuels.6b02681
Modrzejewska-Sikorska A, Konowal E, Klapiszewski L, Nowaczyk G, Jurga S, Jesionowski T, Milczarek G (2017) Lignosulfonate-stabilized selenium nanoparticles and their deposition on spherical silica. Int J Biol Macromol 103:403–408. https://doi.org/10.1016/j.ijbiomac.2017.05.083
Duval A, Molina-Boisseau S, Chirat C (2015) Fractionation of lignosulfonates: comparison of ultrafiltration and ethanol solubility to obtain a set of fractions with distinct properties. Holzforschung 69(2):127–134. https://doi.org/10.1515/hf-2014-0082
Myrvold BO (2013) Salting-out and salting-in experiments with lignosulfonates (LSs). Holzforschung 67(5):549–557. https://doi.org/10.1515/hf-2012-0163
Lou HM, Lai HR, Wang MX, Pang YX, Yang DJ, Qiu XQ, Wang B, Zhang HB (2013) Preparation of lignin-based superplasticizer by graft sulfonation and investigation of the dispersive performance and mechanism in a cementitious system. Ind Eng Chem Res 52(46):16101–16109. https://doi.org/10.1021/ie402169g
Rodriguez-Lucena P, Benedicto A, Lucena JJ, Rodriguez-Castrillon JA, Moldovan M, Alonso JIG, Hernandez-Apaolaza L (2011) Use of the stable isotope Fe-57 to track the efficacy of the foliar application of lignosulfonate/Fe3+ complexes to correct Fe deficiencies in cucumber plants. J Sci Food Agric 91(3):395–404. https://doi.org/10.1002/jsfa.4197
Beatson RP (1992) Determination of sulfonate groups and total sulfur. Springer, Berlin Heidelberg. https://doi.org/10.1177/004947559202200419
Ewing GW (1990) Analytical instrumentation handbook. Marcel Dekker, New York
Loon WMGMV, Boon JJ, Groot BD (1993) Quantitative analysis of sulfonic acid groups in macromolecular lignosulfonic acids and aquatic humic substances by temperature-resolved pyrolysis-mass spectrometry. Environ Sci Technol 27(12):2387–2396. https://doi.org/10.1021/es00048a012
Kennedy JF, Shimizu J (1993) A functional analysis of lignins and their derivatives. Carbohydr Polym 29(1):51–59. https://doi.org/10.1016/0144-8617(96)87021-4
Stranel O, Sebok T (1999) Relationships between the properties of ligninsulphonates and parameters of modified samples with cement binders Part III. Determination of sulphonated compounds content, characteristic of sulphonation, sorption studies. Cem Concr Res 29(11):1769–1772. https://doi.org/10.1016/S0008-8846(99)00165-9
Shul’ga NV, Gomolko LA, Krut’ko NP (2008) Potentiometric titration of lignosulfonic acids. Russ J Appl Chem 81(7):1278–1282. https://doi.org/10.1134/S107042720807029X
Pang YX, Yang DJ, Qiu XQ, Zhang NN (2006) An improvement on the measuring method of the sulphonation degree of lignosulfonate. China Pulp & Paper Industry 27(11):38–40. https://doi.org/10.3969/j.issn.1007-9211.2006.11.012 (In Chinese)
Yang YQ, Zhong-Zheng LI (2003) Study on preparation of dye dispersant by modified craft lignin. Chem Ind For Prod 23(4):31–36. https://doi.org/10.3321/j.issn:0253-2417.2003.04.007 (In Chinese)
Korntner P, Schedl A, Sumerskii I, Zweckmair T, Mahler AK, Rosenau T, Potthast A (2018) Sulfonic acid group determination in lignosulfonates by headspace gas chromatography. ACS Sustain Chem Eng 6(5):6240–6246. https://doi.org/10.1021/acssuschemeng.8b00011
Ratinac KR, Standard OC, Bryant PJ (2004) Lignosulfonate adsorption on and stabilization of lead zirconate titanate in aqueous suspension. J Colloid Interface Sci 273(2):442–454. https://doi.org/10.1016/j.jcis.2004.02.044
Ma'ruf A, Pramudono B, Aryanti N (2018) Synthesis of natural surfactant of sodium lignosulfonate from rice husk lignin by ultrasound assisted - sulfonation. Key Eng Mater 775:20–25. https://doi.org/10.4028/www.scientific.net/KEM.775.20
Funding
The authors acknowledge the financial supports of the National Natural Science Foundation of China (21676109, 21878112), Science and Technology Program of Guangzhou (201707020025), Guangdong Special Support Plan (2016TX03Z298), and Science and Technology Program of Guangdong (2017B090903003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Q., Zeng, M., Zhu, D. et al. A Simple and Rapid Method to Determine Sulfonation Degree of Lignosulfonates. Bioenerg. Res. 12, 260–266 (2019). https://doi.org/10.1007/s12155-019-09972-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-019-09972-x