Abstract
The incentives for utilizing a versatile range of renewable feedstocks in novel ways are continuously increasing. Sulfated polysaccharides from green algae, such as ulvan, are interesting due to the rare sugar constituents which can be utilized for new materials and chemicals in industry. However, before valorization fractionation needs to be performed in a controlled way. In the current work, the kinetics of the aqueous extraction of ulvan was studied in the temperature range 60–130 °C. The highest yield of 97.6 wt.% was attained after 2 h of extraction at 130 °C, and the extraction efficiency was observed to be heavily temperature dependent. Interestingly, two regimes of extraction kinetics were observed, presumably due to the different ulvan fractions contained within the cell wall of green algae. The experimental data was modeled with first-order kinetics, and an apparent activation energy of 53.8 kJ mol−1 was obtained for the process. The algal residue was processed using simultaneous saccharification and fermentation, and 0.48 g ethanol g−1 of sugars was obtained.






Similar content being viewed by others
References
FAO (2015) Pulp and paper capacities, 2014–2019. FAO, Rome
OECD, FAO (2015) OECD-FAO agricultural outlook 2015–2024. doi:10.1787/agr_outlook-2015-en
Lange L, Björnsdóttir B, Brandt A, et al (2015) Development of the Nordic bioeconomy-NMC reporting: test centers for green energy solutions—biorefineries and business needs. doi:10.6027/TN2015-582
Harun R, Yip JWS, Thiruvenkadam S et al (2014) Algal biomass conversion to bioethanol—a step-by-step assessment. Biotechnol J 9:73–86. doi:10.1002/biot.201200353
Percival E (1979) The polysaccharides of green, red and brown seaweeds: their basic structure, biosynthesis and function. Br Phycol J 14:103–117. doi:10.1080/00071617900650121
Jiao G, Yu G, Zhang J, Ewart HS (2011) Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Drugs 9:196–233. doi:10.3390/md9020196
Paradossi G, Cavalieri F, Chiessi E (2002) A conformational study on the algal polysaccharide ulvan. Macromolecules 35:6404–6411. doi:10.1021/ma020134s
Wang L, Wang X, Wu H, Liu R (2014) Overview on biological activities and molecular characteristics of sulfated polysaccharides from marine green algae in recent years. Mar Drugs 12:4984–5020. doi:10.3390/md12094984
Cunha L, Grenha A (2016) Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar Drugs 14:42. doi:10.3390/md14030042
Chiellini F, Morelli A (2011) Ulvan: a versatile platform of biomaterials from renewable resources. In: Pignatello R (ed) Biomater. - Phys. Chem. InTech, Rijeka, pp 75–98
Ray B, Lahaye M (1995) Cell-wall polysaccharides from the marine green alga Ulva “rigida” (Ulvales, Chlorophyta). Extraction and chemical composition. Carbohydr Res 274:251–261. doi:10.1016/0008-6215(95)00138-J
Siddhanta AK, Goswami AM, Ramavat BK et al (2001) Water soluble polysaccharides of marine algal species of Ulva (Ulvales, Chlorophyta) of Indian waters. Indian J Mar Sci 30:166–172
Toskas G, Hund RD, Laourine E et al (2011) Nanofibers based on polysaccharides from the green seaweed Ulva rigida. Carbohydr Polym 84:1093–1102. doi:10.1016/j.carbpol.2010.12.075
Balboa EM, Soto ML, Nogueira DR et al (2014) Potential of antioxidant extracts produced by aqueous processing of renewable resources for the formulation of cosmetics. Ind Crop Prod 58:104–110. doi:10.1016/j.indcrop.2014.03.041
Pezoa-Conte R, Leyton A, Anugwom I et al (2015) Deconstruction of the green alga Ulva rigida in ionic liquids: closing the mass balance. Algal Res 12:262–273. doi:10.1016/j.algal.2015.09.011
Buschmann AHA, Gonzalez MDCH, Varela D (2008) Seaweed future cultivation in Chile: perspectives and challenges. Int J Environ Pollut 33:432. doi:10.1504/IJEP.2008.020571
Camus C, Buschmann AH (2014) Aquaculture in Chile: what about seaweeds? 40–42
van der Burg S, Stuiver M, Veenstra F, Bikker P, Lopez Contreras A, Palstra A, Broeze J, Jansen H, Jak R, Gerritsen A, Harmsen P, Kals J, Blanco A, Branderburg W, van Krimpen M, van Duijn AP, Mulder W, van Raamsdonk L (2013) Triple P review of the feasibility of sustainable offshore seaweed production in the North Sea, Wageningen, Wageningen UR (University and Research centre), LEI Report 13-077, pp. 106
Vasquez JA, Camus P, Ojeda FP (1998) Diversidad, estructura y funcionamiento de ecosistemas costeros rocosos del norte de Chile. Rev Chil Hist Nat 71:479–499
Camus PA (2008) Diversidad, distribución y abundancia de especies en ensambles intermareales rocosos. Rev Biol Mar Oceanogr 43:615–627. doi:10.4067/S0718-19572008000300021
Stiger-Pouvreau V, Bourgougnon N, Deslandes E (2016) Carbohydrates from seaweeds. In: Levine IA, Fleurence J (eds) Seaweed Heal. Dis. Prev., 1st edn. Academic Press, London, pp 223–274
Wageningen UR (2015) Farming at sea. Resource 10:31
Carl C, De Nys R, Paul NA (2014) The seeding and cultivation of a tropical species of filamentous Ulva for algal biomass production. PLoS One. doi:10.1371/journal.pone.0098700
Pinchetti JLG, Del Campo FE, Moreno Díez P, García Reina G (1998) Nitrogen availability influences the biochemical composition and photosynthesis of tank-cultivated Ulva rigida (Chlorophyta). J Appl Phycol 10:383–389. doi:10.1023/A:1008008912991
Dawe C (2016) Macroalgae systematics. In: Fleurence J, Levine I (eds) Seaweed Heal. Dis. Prev., 1st edn. Academic Press, London, pp 107–148
Karray R, Hamza M, Sayadi S (2015) Evaluation of ultrasonic, acid, thermo-alkaline and enzymatic pre-treatment on anaerobic digestion of Ulva rigida for biogas production. Bioresour Technol 187:205–213. doi:10.1016/j.biortech.2015.03.108
van der Wal H, Sperber BLHM, Houweling-Tan B et al (2013) Production of acetone, butanol, and ethanol from biomass of the green seaweed Ulva lactuca. Bioresour Technol 128:431–437. doi:10.1016/j.biortech.2012.10.094
Karray R, Hamza M, Sayadi S (2016) Production and characterization of enzymatic cocktail produced by Aspergillus niger using green macroalgae as nitrogen source and its application in the pre-treatment for biogas production from Ulva rigida. Bioresour Technol 216:622–628. doi:10.1016/j.biortech.2016.05.067
Kim NJ, Li H, Jung K et al (2011) Ethanol production from marine algal hydrolysates using Escherichia coli KO11. Bioresour Technol 102:7466–7469. doi:10.1016/j.biortech.2011.04.071
Grénman H, Eränen K, Krogell J et al (2011) Kinetics of aqueous extraction of hemicelluloses from spruce in an intensified reactor system. Ind Eng Chem Res 50:3818–3828. doi:10.1021/ie101946c
Rissanen JV, Grénman H, Willför S et al (2014) Spruce hemicellulose for chemicals using aqueous extraction: kinetics, mass transfer, and modeling. Ind Eng Chem Res 53:6341–6350. doi:10.1021/ie500234t
Rissanen JV, Grénman H, Xu C et al (2014) Obtaining spruce hemicelluloses of desired molar mass by using pressurized hot water extraction. ChemSusChem 7:2947–2953. doi:10.1002/cssc.201402282
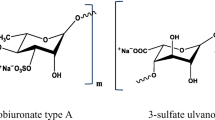
Lahaye M, Robic A (2007) Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 8:24–30. doi:10.1021/bm061185q
Lahaye M, Ray B (1996) Cell-wall polysaccharides from the marine green alga Ulva “rigida” (Ulvales, Chlorophyta)—NMR analysis of ulvan oligosaccharides. Carbohydr Res 283:161–173. doi:10.1016/0008-6215(95)00407-6
Willför S, Pranovich A, Tamminen T et al (2009) Carbohydrate analysis of plant materials with uronic acid-containing polysaccharides—a comparison between different hydrolysis and subsequent chromatographic analytical techniques. Ind Crop Prod 29:571–580. doi:10.1016/j.indcrop.2008.11.003
Shuuluka D, Bolton JJ, Anderson RJ (2013) Protein content, amino acid composition and nitrogen-to-protein conversion factors of Ulva rigida and Ulva capensis from natural populations and Ulva lactuca from an aquaculture system, in South Africa. J Appl Phycol 25:677–685. doi:10.1007/s10811-012-9902-5
Ray B, Lahaye M (1995) Cell-wall polysaccharides from the marine green alga “Ulva rigida” (Ulvales, Chlorophyta). Chemical structure of ulvan. Carbohydr Res 274:313–318
Robic A, Gaillard C, Sassi JF et al (2009) Ultrastructure of ulvan: a polysaccharide from green seaweeds. Biopolymers 91:652–664. doi:10.1002/bip.21195
Robic A, Sassi JF, Lahaye M (2008) Impact of stabilization treatments of the green seaweed Ulva rotundata (Chlorophyta) on the extraction yield, the physico-chemical and rheological properties of ulvan. Carbohydr Polym 74:344–352. doi:10.1016/j.carbpol.2008.02.020
Percival E, McDowell RH (1968) Chemistry and enzymology of marine algal polysaccharides. Sci Prog 56:283–285. doi:10.1002/ange.19680802022
Yaich H, Garna H, Besbes S et al (2013) Effect of extraction conditions on the yield and purity of ulvan extracted from Ulva lactuca. Food Hydrocoll 31:375–382. doi:10.1016/j.foodhyd.2012.11.013
Costa C, Alves A, Pinto PR et al (2012) Characterization of ulvan extracts to assess the effect of different steps in the extraction procedure. Carbohydr Polym 88:537–546. doi:10.1016/j.carbpol.2011.12.041
Alves A, Sousa RA, Reis RL (2013) A practical perspective on ulvan extracted from green algae. J Appl Phycol 25:407–424. doi:10.1007/s10811-012-9875-4
Turvey JR (1965) Sulfates of the simple sugars. Adv Carbohydr Chem 20:183–218. doi:10.1016/S0096-5332(08)60299-4
Percival E (1980) Desulfation of polysaccharides. Methods Carbohydr Chem 8:281–285. doi:10.1016/B978-0-12-746208-0.50048-7
Andrieux C, Hibert A, Houari AM et al (1998) Ulva lactuca is poorly fermented but alters bacterial metabolism in rats inoculated with human faecal flora from methane and non-methane producers. J Sci Food Agric 77:25–30. doi:10.1002/(SICI)1097-0010(199805)77:1<25::AID-JSFA989>3.0.CO;2-C
Bobin-Dubigeon C, Lahaye M, Guillon F et al (1997) Factors limiting the biodegradation of Ulva sp. cell-wall polysaccharides. J Sci Food Agric 75:341–351. doi:10.1002/(SICI)1097-0010(199711)75:3<341::AID-JSFA888>3.0.CO;2-B
Vauchel P, Leroux K, Kaas R et al (2009) Kinetics modeling of alginate alkaline extraction from Laminaria digitata. Bioresour Technol 100:1291–1296. doi:10.1016/j.biortech.2008.03.005
Rissanen JV, Murzin DY, Salmi T, Grénman H (2016) Aqueous extraction of hemicelluloses from spruce—from hot to warm. Bioresour Technol 199:279–282. doi:10.1016/j.biortech.2015.08.116
Vallejos ME, Felissia FE, Kruyeniski J, Area MC (2015) Kinetic study of the extraction of hemicellulosicbohydrates from sugarcane bagasse by hot water treatment. Ind Crop Prod 67:1–6. doi:10.1016/j.indcrop.2014.12.058
Leyton A, Pezoa-Conte R, Barriga A et al (2016) Identification and efficient extraction method of phlorotannins from the brown seaweed Macrocystis pyrifera using an orthogonal experimental design. Algal Res 16:201–208. doi:10.1016/j.algal.2016.03.019
Yanomoto M (1980) Physicochemical studies on sulfated polysaccharides extracted from seaweeds at various temperatures. Agric Biol Chem 44:589–593. doi:10.1080/00021369.1980.10863990
Mittal A, Chatterjee SG, Scott GM, Amidon TE (2009) Modeling xylan solubilization during autohydrolysis of sugar maple wood meal: reaction kinetics. Holzforschung 63:307–314. doi:10.1515/HF.2009.054
Nabarlatz D, Farriol X, Montané D (2004) Kinetic modeling of the autohydrolysis of lignocellulosic biomass for the production of hemicellulose-derived oligosaccharides. Ind Eng Chem Res 43:4124–4131. doi:10.1021/ie034238i
Acknowledgements
This work is a part of the activities of the Johan Gadolin Process Chemistry Centre (PCC), a Centre of Excellence financed by Åbo Akademi University. Also, the Bio4Energy program and the Wallenberg Wood Science Center are acknowledged. This work was partially financed by the Academy of Finland (AKA) (Grant Number 268937), the National Commission for Scientific and Technologic Research of the Government of Chile (CONICYT, Project AKA-ERNC 0009) and the Centre for Biotechnology and Bioengineering (CeBiB) FB-0001. Prof. Johan Bobacka, Sten Lindholm, and Luis Bezerra from PCC are acknowledged for their collaboration with ion-exchange chromatograph and the nitrogen content analysis. Prof. Mario Eddning from Northern Catholic University of Chile is acknowledged for providing the algal samples. Ricardo Pezoa-Conte gratefully acknowledges the CONICYT/Becas Chile for the scholarship (No. 72170085) provided to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pezoa-Conte, R., Leyton, A., Baccini, A. et al. Aqueous Extraction of the Sulfated Polysaccharide Ulvan from the Green Alga Ulva rigida—Kinetics and Modeling. Bioenerg. Res. 10, 915–928 (2017). https://doi.org/10.1007/s12155-017-9853-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-017-9853-4