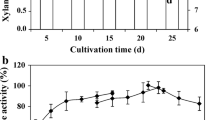
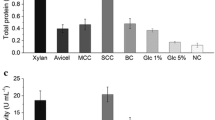
Abstract
A Mexican strain of Talaromyces stollii LV186 was isolated from decaying pretreated corn stover. The production of cellulase and xylanase enzyme cocktails was evaluated with corn and sorghum stover used as inducers in a mineral medium. The volumetric and specific activities of T. stollii LV186 were compared with the values produced by Trichoderma reesei ATCC 26921 in a time-course experiment. After the submerged culture and a posterior ultrafiltration stage, the enzyme complexes were evaluated over acid-pretreated corn or sorghum stover in baffled flasks under controlled temperature and agitation conditions, and hydrolysis levels of 30 and 39 % of the theoretical maximum were obtained after only 72-h reactions, for each substrate. A side-by-side comparison showed a better ratio of endoglucanase to cellobiohydrolase to β-glucosidase and of xylanase to β-xylosidase enzymes in T. stollii than in T. reesei ATCC 26921. Furthermore, the hydrolysis of pretreated corn and sorghum stover achieved by T. stollii is significantly higher compared with that of a commercial cocktail from T. reesei ATCC 26921 (Celluclast). Therefore, the T. stollii LV186 strain is a good candidate for the hydrolysis of complex lignocellulose substrates. To the authors’ knowledge, this study is the first to describe the cellulolytic and hemicellulolytic activities produced by a T. stollii strain.






Similar content being viewed by others
References
Alvira P, Tomás-Pejó E, Ballesteros MJ, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101:4851–4861. doi:10.1016/j.biortech.2009.11.093
Goldbeck R, Ramos MM, Pereira GAG, Maugeri-Filho F (2013) Cellulase production from a new strain Acremonium strictum isolated from the Brazilian biome using different substrates. Bioresour Technol 128:797–803. doi:10.1016/j.biortech.2012.10.034
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83(1):1–11. doi:10.1016/S0960-8524(01)00212-7
Lal R (2009) Soil quality impacts of residue removal for bioethanol production. Soil Till Res 102:233–241. doi:10.1016/j.still.2008.07.003
SIAP (2014) Servicio de Información Agroalimentaria y Pesquera. http://www.siap.gob.mx. Accessed December 2015
Vargas-Tah A, Moss-Acosta C, Trujillo Martinez B, Tiessen A, Lozoya-Gloria E, Orencio-Trejo M, Gosset G, Martinez A (2015) Non-severe thermochemical hydrolysis of stover from white corn and sequential enzymatic saccharification and fermentation to ethanol. Bioresource Technol 198:611–618. doi:10.1016/j.biortech.2015.09.036
Badhan AK, Chadra B, Kaur J, Saini HS, Bhat MK (2007) Production of multiple xylanolytic and cellulolytic enzymes by thermophilic fungus Myceliophthora sp IMI 387099. Bioresour Technol 98:504–510. doi:10.1016/j.biortech.2006.02.009
Van den Brink J, De Vries RP (2011) Fungal enzyme sets for plant polysaccharide degradation. Appl Microbiol Biotechnol 91:1477–1492. doi:10.1007/s00253-011-3473-2
Shi Q-Q, Sun J, H-L Y, Li C-X, Bao J, J-H X (2011) Catalytic performance of corn stover hydrolysis by a new isolate Penicillium sp. ECU0913 producing both cellulase and xylanase. Appl Biochem Biotechnol 164:819–830. doi:10.1007/s12010-011-9176-4
Castro AM, Lins de Albuquerque de Carvalho M, Gomes Ferreira Leite S, Pereira N Jr (2010) Cellulases from Penicillium funiculosum: production, properties and application to cellulose hydrolysis. J Ind Microbiol Biotechnol 37:151–158. doi:10.1007/s10295-009-0656-2
Sørensen A, Teller PJ, Lübeck PS, Ahring BK (2011) Onsite enzyme production during bioethanol production from biomass: screening for suitable fungal strains. Appl Biochem Biotechnol 164:1058–1070. doi:10.1007/s12010-011-9194-2
Delabona PDS, Farinas CS, da Silva MR, Azzoni FS, Pradella JGC (2012) Use of a new Trichoderma harzianum strain isolated from the Amazon rainforest with pretreated sugar cane bagasse for on-site cellulase production. Bioresour Technol 107:517–521. doi:10.1016/j.biortech.2011.12.048
Benjamin CR (1955) Ascocarps of Aspergillus and Penicillium. Mycology 47:669–687
Berbee ML, Yoshimura A, Sugiyama J, Taylor JW (1995) Is Penicillium monophyletic? An evaluation of phylogeny in the family Trichocomaceae from 18S, 5.8S and ITS ribosomal DNA sequence data. Mycology 87:210–222. http://www.jstor.org/stable/3760907
Sang H, An TJ, Kim CS, Shin G-S, Sung G-H, Yu SH (2013) Two novel Talaromyces species isolated from medicinal crops in Korea. J Microbiol 51(5):704–708. doi:10.1007/s12275-013-3361-9
Peterson SW, Jurjević Ž (2013) Talaromyces columbinus sp. nov., and genealogical concordance analysis in Talaromyces clade 2a. PLoS One 8:e78084. doi:10.1371/journal.pone.0078084
Moloney AP, Considine PJ, Coughlan MP (1983) Cellulose hydrolysis by the cellulases produced by Talaromyces emersonii when grown on different inducing substrates. Biotechnol Bioeng 25:1169–1173. doi:10.1002/bit.260250423
Moloney AP, McCrae SI, Wood TM, Coughlan MP (1985) Isolation and characterization of the 1,4-β-D-glucan grucanohydrolases of Talaromyces emersonii. Biochem J 225:365–374
McCarthy T, Hanniffy O, Savage AV, Tuohy MG (2003) Catalytic properties and mode of action of three endo-β-glucanases from Talaromyces emersonii on soluble β-1,4- and β-1,3;1,4-linked glucans. Int J Biol Macromol 33:141–148. doi:10.1016/S0141-8130(03)00080-1
Tuohy MG, Walsh DJ, Murray PG, Claeyssens M, Cuffe MM, Savage AV, Coughlan MP (2002) Kinetic parameters and mode of action of the cellobiohydrolases produced by Talaromyces emersonii. Biochim Biophys Acta 1596:366–380. doi:10.1016/S0167-4838(01)00308-9
Collins CM, Murray PG, Denman S, Morrissey JP, Byrnes L, Teeri TT, Tuohy M (2007) Molecular cloning and expression analysis of two distinct β-glucosidase genes, bg1 and aven1, with very different biological roles from the thermophilic, saprophytic fungus Talaromyces emersonii. Mycol Res 111:840–849. doi:10.1016/j.mycres.2007.05.007
Voutilainen SP, Murray PG, Tuohy MG, Koivula A (2010) Expression of Talaromyces emersonii cellobiohydrolase Cel7A in Saccharomyces cerevisiae and rational mutagenesis to improve its thermostability and activity. Protein Eng Des Sel 23:69–79. doi:10.1093/protein/gzp072
Fujii T, Inoue H, Ashikawa K (2015) Decreased cellulase and xylanase production in the fungus Talaromyces cellulolyticus by disruption of tacA and tctA genes, encoding putative zinc finger transcriptional factors. Appl Biochem Biotechnol 175:3218–3229. doi:10.1007/s12010-015-1497-2
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Determination of structural carbohydrates and lignin in biomass. Technical Report. NREL/TP-510-42618. Available from: (http://www.nrel.gov/biomass/analytical_procedures.html).
Sluiter A, Ruiz R, Scarlata C, Sluiter J, Templeton D (2008) Determination of extractives in biomass. Technical report. NREL/TP-510-42619. Available from: (http://www.nrel.gov/biomass/analytical_procedures.html).
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2008) Determination of ash in biomass. Technical Report NREL/TP-510-42622. Available from (http://www.nrel.gov/biomass/analytical_procedures.html).
Mandels M, Weber J (1969) The production of cellulases. Adv Chem Ser 95:394–414
Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque A, Chen W (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. PNAS 109:6241–6246. doi:10.1073/pnas.1117018109
Ebenhardt U (2012) Methods for DNA barcoding of fungi. In: Kress WJ, Erickson DL (eds) DNA barcodes: methods and protocols, methods in molecular biology, vol 858. Springer Science Business Media, pp. 183–205. doi:10.1007/978-1-61779-591-6_9
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi:10.1093/molbev/msr121
Samson RA, Yilmaz N, Houbraken J, Spierenburg H, Seifert KA, Peterson SW, Varga J, Frisvad JC (2011) Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud Mycol 70:159–183. doi:10.3114/sim.2011.70.04
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10(3):512–526
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. doi:10.1385/0-89603-268-X:9
Teather RM, Wood PJ (1982) Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol 43:777–780
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268. doi:10.1351/pac198759020257
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. doi:10.1021/ac60147a030
Wood TM, Bhat KM (1988) Methods for measuring cellulase activities. Methods Enzymol 160:87–112
Ghose TK, Bisaria VS (1987) Measurement of hemicellulase activities. Pure Appl Chem 59:1739–1752. doi:10.1351/pac198759121739
Grassick A, Birrane G, Tuohy M, Murray P, Higgins T (2003) Crystallization and preliminary crystallographic analysis of the catalytic domain cellobiohydrolase I from Talaromyces emersonii. Acta Crystallogr Sect D Biol Crystallogr 59:1283–1284. doi:10.1107/S0907444903009843
Nakkharat P, Haltrich D (2006) Purification and characterization of an intracellular enzyme with β-glucosidase and β-galactosidase activity from the thermophilic fungus Talaromyces thermophilus CBS 236.58. J Biotechnol 123:304–313. doi:10.1016/j.jbiotec.2005.12.015
Mandalari G, Bisignano G, Lo Curto RB, Waldron KW, Faulds CB (2008) Production of feruloyl esterases and xylanases by Talaromyces stipitatus and Humicola grisea var. thermoidea on industrial food processing by-products. Bioresour Technol 99:5130–5133. doi:10.1016/j.biortech.2007.09.022
Maalej I, Belhaj I, Masmoudi NF, Belghith H (2009) Highly thermostable xylanase of the thermophilic fungus Talaromyces thermophiles: purification and characterization. Appl Biochem Biotechnol 158:200–212. doi:10.1007/s12010-008-8317-x
Adsul MG, Bastawde KB, Varma AJ, Gokhale DV (2007) Strain improvement of Penicillium janthinellum NCIM 1171 for increased cellulase production. Bioresour Technol 98:1467–1473. doi:10.1016/j.biortech.2006.02.036
Camassola M, Dillon AJP (2009) Biological pretreatment of sugar cane bagasse for the production of cellulases and xylanases by Penicillium echinulatum. Ind Crop Prod 29:642–647. doi:10.1016/j.indcrop.2008.09.008
Vimala Rodhe A, Sateesh L, Sridevi J, Venkateswarlu B, Venkateswar R (2011) Enzymatic hydrolysis of sorghum straw using native cellulase produced by T. reesei NCIM 992 under solid state fermentation using rice straw. 3. Biotech 1:207–215. doi:10.1007/s13205-011-0024-6
Bischof R, Fourtis L, Limbeck A, Gamauf C, Seiboth B, Kubicek CP (2013) Comparative analysis of the Trichoderma reesei transcriptome during growth on the cellulase inducing substrates wheat straw and lactose. Biotechnol Biofuels 6:127. doi:10.1186/1754-6834-6-127
Martins LF, Kolling D, Camassola M, Dillon AJP, Ramos LP (2008) Comparison of Penicillium echinulatum and Trichoderma reesei cellulases in relation to their activity against various cellulosic substrates. Bioresour Technol 99(5):1417–1424. doi:10.1016/j.biortech.2007.01.060
Castro AM, Pedro KCNR, da Cruz JC, Ferreira MC, Leite SGF, Pereira N (2010) Trichoderma harzianum IOC-4038: a promising strain for the production of a cellulolytic complex with significant β-glucosidase activity from sugarcane bagasse cellulignin. Appl Biochem Biotechnol 162:2111–2122. doi:10.1007/s12010-010-8986-0
Krogh KBR, Mørkeberg A, Jørgensen H, Frisvad JC, Olsson L (2004) Screening genus Penicillium for producers of cellulolytic and xylanolytic anzymes. Appl Biochem Biotechnol 113-116:389–401
Dinis M, Bezerra R, Nunes F, Dias AA, Guedes CV, Ferreira LMM, Cone JW, Marques GSM, Barros ARN, Rodrigues MAM (2009) Modification of wheat straw lignin by solid state fermentation. Bioresour Technol 100:4829–4835. doi:10.1016/j.biortech.2009.04.036
Karboune S, Geraert PA, Kermasha S (2008) Characterization of selected cellulolytic activities of multi-enzymatic complex system from Penicillium funiculosum. J Agric Food Chem 56:903–909. doi:10.1021/jf072847l
Lu Y, Wang Y, Xu G, Chu J, Zhuang Y, Zhang S (2010) Influence of high solid concentration on enzymatic hydrolysis and fermentation of steam-exploded corn stover biomass. Appl Biochem Biotechnol 160:360–369. doi:10.1007/s12010-008-8306-0
Ximenes E, Kim Y, Mosier N, Dien B, Ladisch M (2010) Inhibition of cellulases by phenols. Enzym Microb Technol 46:170–176. doi:10.1016/j.enzmictec.2009.11.001
Chandra RP, Bura R, Mabee WE, Berlin A, Pan X, Sddler JN (2007) Substrate pretreatment: the key to effective enzymatic hydrolysis of lignocellulosics? Adv Biochem Engin/Biotecnol 108:67–93. doi:10.1007/10_2007_064
Kumar R, Singh S, Singh OV (2008) Bioconversion of lignocellulosic biomass: biochemical and molecular perspectives. J Ind Microbiol Biotechnol 35:377–391. doi:10.1007/s10295-008-0327-8
Acknowledgments
The authors thank the Alcoholera del Centro S.A. de C.V. and The Mexican Council of Science and Technology (CONACyT) Technological Innovation, grants PETRAMIN 2010-138079, 2011-154298, and PETRAMIN/IDESA 2012-184417 and Secretaría de Energía (SENER)-CONACYT project 151029 to Centro de Investigaciones y de Estudios Avanzados del Instituto Politécnico Nacional. We thank Mario A. Caro-Bermudez (Biotechnology Institute, Universidad Nacional Autónoma de México), for providing us the pretreated materials and the Bioenergy Thematic Network (“Red Temática de Bioenergía”) grant 260457.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Orencio-Trejo, M., Torres-Granados, J., Rangel-Lara, A. et al. Cellulase and Xylanase Production by the Mexican Strain Talaromyces stollii LV186 and Its Application in the Saccharification of Pretreated Corn and Sorghum Stover. Bioenerg. Res. 9, 1034–1045 (2016). https://doi.org/10.1007/s12155-016-9791-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-016-9791-6