Abstract
Objectives
There has been developed a clinical dynamic total-body 68Ga-DOTATATE PET/CT imaging protocol that allows quantitative imaging of net influx rate (Ki). Using qualitative and quantitative analyses of clinical studies, this retrospective study aims to assess whether parametric Ki images improve lesion detectability.
Methods
Using a 194-cm axial field-of-view PET/CT scanner, 52 patients with neuroendocrine tumors underwent a 60-min dynamic total-body 68Ga-DOTATATE scan. Parametric Ki images and static standardized uptake value (SUV) images were generated. In addition to visual inspection of both sets of images, a quantitative analysis of 249 individual lesions was conducted using the target-to-background (TBR) metric.
Results
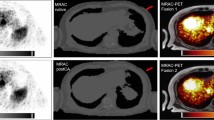
There were 52 patients who underwent dynamic total-body 68Ga-DOTATATE PET/CT scans. A total of 249 lesions were evaluated, of which 66 lesions were biopsy-proven and 183 lesions were unproven. Ki images produced two fewer false positives than the SUV images. Overall, our results from 66 proven NET lesions suggested similar sensitivity (98.5%) but improved accuracy (from 95.6 to 97.1%) and potentially enhanced specificity with Ki over SUV imaging. Besides, there was no difference in the number of pathological lesions identified visually in both images. However, Ki TBR was significantly higher than SUV TBR quantitatively (P < 0.001).
Conclusions
Patlak Ki imaging provides nuclear physicians with a PET image with higher tumor contrast which may enhance confidence in diagnosis with possibly reduced false positive results, albeit an equivalent detectability, compared to static SUV image.





Similar content being viewed by others
Data availability
Data are available based on the reasonable request to the corresponding author.
References
Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–42.
Sundin A, Rockall A. Therapeutic monitoring of gastroenteropancreatic neuroendocrine tumors: the challenges ahead. Neuroendocrinology. 2012;96:261–71.
Haug AR, Auernhammer CJ, Wangler B, Schmidt GP, Uebleis C, Goke B, et al. 68Ga-DOTATATE PET/CT for the early prediction of response to somatostatin receptor-mediated radionuclide therapy in patients with well-differentiated neuroendocrine tumors. J Nucl Med. 2010;51:1349–56.
Gabriel M, Oberauer A, Dobrozemsky G, Decristoforo C, Putzer D, Kendler D, et al. 68Ga-DOTA-Tyr3-octreotide PET for assessing response to somatostatin-receptor-mediated radionuclide therapy. J Nucl Med. 2009;50:1427–34.
Velikyan I, Sundin A, Sorensen J, Lubberink M, Sandstrom M, Garske-Roman U, et al. Quantitative and qualitative intrapatient comparison of 68Ga-DOTATOC and 68Ga-DOTATATE: net uptake rate for accurate quantification. J Nucl Med. 2014;55:204–10.
Wang G, Qi J. Direct estimation of kinetic parametric images for dynamic PET. Theranostics. 2013;3:802–15.
Veronese M, Rizzo G, Bertoldo A, Turkheimer FE. Spectral analysis of dynamic PET studies: a review of 20 years of method developments and applications. Comput Math Methods Med. 2016;2016:7187541.
Ilan E, Sandstrom M, Velikyan I, Sundin A, Eriksson B, Lubberink M. Parametric net influx rate images of 68Ga-DOTATOC and 68Ga-DOTATATE: quantitative accuracy and improved image contrast. J Nucl Med. 2017;58:744–9.
Liu G, Hu P, Yu H, Tan H, Zhang Y, Yin H, et al. Ultra-low-activity total-body dynamic PET imaging allows equal performance to full-activity PET imaging for investigating kinetic metrics of 18F-FDG in healthy volunteers. Eur J Nucl Med Mol Imaging. 2021;48:2373–83.
Liu G, Xu H, Hu P, Tan H, Zhang Y, Yu H, et al. Kinetic metrics of 18F-FDG in normal human organs identified by systematic dynamic total-body positron emission tomography. Eur J Nucl Med Mol Imaging. 2021;48:2363–72.
Zhang X, Xie Z, Berg E, Judenhofer MS, Liu W, Xu T, et al. Total-Body Dynamic Reconstruction and Parametric Imaging on the uEXPLORER. J Nucl Med. 2020;61:285–91.
Dias AH, Pedersen MF, Danielsen H, Munk OL, Gormsen LC. Clinical feasibility and impact of fully automated multiparametric PET imaging using direct Patlak reconstruction: evaluation of 103 dynamic whole-body 18F-FDG PET/CT scans. Eur J Nucl Med Mol Imaging. 2021;48:837–50.
Fahrni G, Karakatsanis NA, Di Domenicantonio G, Garibotto V, Zaidi H. Does whole-body Patlak 18F-FDG PET imaging improve lesion detectability in clinical oncology? Eur Radiol. 2019;29:4812–21.
Sari H, Mingels C, Alberts I, Hu J, Buesser D, Shah V, et al. First results on kinetic modelling and parametric imaging of dynamic 18F-FDG datasets from a long axial FOV PET scanner in oncological patients. Eur J Nucl Med Mol Imaging. 2022;49:1997–2009.
Breeman WA, de Jong M, de Blois E, Bernard BF, Konijnenberg M, Krenning EP. Radiolabelling DOTA-peptides with 68Ga. Eur J Nucl Med Mol Imaging. 2005;32:478–85.
Karakatsanis NA, Lodge MA, Tahari AK, Zhou Y, Wahl RL, Rahmim A. Dynamic whole-body PET parametric imaging: i. Concept, acquisition protocol optimization and clinical application. Phys Med Biol. 2013;58:7391–418.
Dias AH, Jochumsen MR, Zacho HD, Munk OL, Gormsen LC. Multiparametric dynamic whole-body PSMA PET/CT using [68Ga]Ga-PSMA-11 and [18F]PSMA-1007. EJNMMI Res. 2023;13:31.
Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72.
Gangi A, Howe JR. The landmark series: neuroendocrine tumor liver metastases. Ann Surg Oncol. 2020;27:3270–80.
Chamberlain RS, Canes D, Brown KT, Saltz L, Jarnagin W, Fong Y, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000;190:432–45.
Pavel M, Baudin E, Couvelard A, Krenning E, Oberg K, Steinmuller T, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95:157–76.
Kunikowska J, Krolicki L, Pawlak D, Zerizer I, Mikolajczak R. Semiquantitative analysis and characterization of physiological biodistribution of 68Ga-DOTA-TATE PET/CT. Clin Nucl Med. 2012;37:1052–7.
Moradi F, Jamali M, Barkhodari A, Schneider B, Chin F, Quon A, et al. Spectrum of 68Ga-DOTA TATE uptake in patients with neuroendocrine tumors. Clin Nucl Med. 2016;41:e281–7.
Reubi JC, Waser B, Schaer JC, Laissue JA. Somatostatin receptor sst1-sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur J Nucl Med. 2001;28:836–46.
Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev. 2003;24:389–427.
Frilling A, Akerstrom G, Falconi M, Pavel M, Ramos J, Kidd M, et al. Neuroendocrine tumor disease: an evolving landscape. Endocr Relat Cancer. 2012;19:R163–85.
Funding
This study was funded by the National Key Research and Development Program of China (Grant number: 2022YFC2406902 to H.S.), the Shanghai Municipal Key Clinical Specialty Project (Grant number: SHSLCZDZK03401 to H.S.), the Major Science and Technology Projects for Major New Drug Creation (Grant number: 2019ZX09302001 to H.S.), the Shanghai Science and Technology Committee Program (Grant number: 20DZ2201800 to H.S.), the Three-year Action Plan of Clinical Skills and Innovation of Shanghai Hospital Development Center (Grant number: SHDC2020CR3079B to H.S.), and the Next Generation Information Infrastructure Construction Project founded by Shanghai Municipal Commission of Economy and Informatization (Grant number: 201901014 to H.S.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yin, H., Liu, G., Mao, W. et al. Parametric net influx rate imaging of 68Ga-DOTATATE in patients with neuroendocrine tumors: assessment of lesion detectability. Ann Nucl Med (2024). https://doi.org/10.1007/s12149-024-01922-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12149-024-01922-8