Abstract
Objective
The aim of this study was to investigate the potential value of dual tracers 18F-FDG and 18F-FES PET/CT in predicting response to Cyclin-Dependent 4/6 Kinase (CDK4/6) inhibitors combined with endocrine therapy for metastatic estrogen receptor (ER)-positive breast cancer patients.
Methods
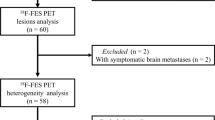
This retrospective study enrolled 38 ER-positive metastatic breast cancer patients from our center who underwent both 18F-FDG and 18F-FES PET/CT scans within 1 month before CDK4/6 inhibitors combined with endocrine therapy. The extracted parameters comprised the maximum standardized uptake value (SUVmax) for both FDG and FES PET, as well as the ratio between FES and FDG SUVmax. Each parameter was dichotomized based on its median threshold. The primary endpoint was progression-free survival (PFS), which was estimated using the Kaplan–Meier method and compared by the log-rank test.
Results
After a median follow-up of 15.6 months, progressive disease was observed in 23 out of 38 patients, and the median PFS for the whole cohort was 21.0 months [95% confidence interval (CI) 12.7–29.3]. FES and FDG PET identified 6 patients (15.8%) with FES-negative lesions, suggesting ER heterogeneity in metastatic lesions. The median PFS of these patients was only 5.3 months (95% CI 1.7–8.9), which was substantially shorter than that of patients with 100% FES-positive lesions (median PFS 22.9 months, 95% CI 17.1–28.7, P < 0.001). Patients with 100% FES-positive lesions who had high FES/FDG showed significantly shorter PFS compared to those with low FES/FDG (14.9 vs. 30.5 months, P = 0.003).
Conclusions
This study shows that FDG and FES PET imaging may serve as valuable tools for patient selection in the context of CDK4/6 inhibitor therapy combined with endocrine treatment, and have the potential to function as prognostic biomarkers.




Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30.
Johnston SJ, Cheung KL. Endocrine therapy for breast cancer: a model of hormonal manipulation. Oncol Ther. 2018;6(2):141–56.
Polk A, Kolmos IL, Kümler I, et al. Specific CDK4/6 inhibition in breast cancer: a systematic review of current clinical evidence. ESMO Open. 2016;1(6):e000093.
Goel S, Bergholz JS, Zhao JJ. Targeting CDK4 and CDK6 in cancer. Nat Rev Cancer. 2022;22(6):356–72.
Spring LM, Wander SA, Andre F, et al. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: past, present, and future. Lancet. 2020;395(10226):817–27.
O’sullivan CC, Clarke R, Goetz MP, et al. Cyclin-dependent kinase 4/6 inhibitors for treatment of hormone receptor-positive, ERBB2-negative breast cancer: a review. JAMA Oncol. 2023;2023:1.
Huppert LA, Gumusay O, Idossa D, et al. Systemic therapy for hormone receptor-positive/human epidermal growth factor receptor 2-negative early stage and metastatic breast cancer. CA Cancer J Clin. 2023;2023:1.
Toi M, Boyle F, Im YH, et al. Adjuvant abemaciclib combined with endocrine therapy: efficacy results in monarche cohort 1. Oncologist. 2023;28(1):e77–81.
Li Y, Li W, Gong C, et al. A multicenter analysis of treatment patterns and clinical outcomes of subsequent therapies after progression on palbociclib in HR+/HER2− metastatic breast cancer. Ther Adv Med Oncol. 2021;13:17588359211022890.
Wang B, Li R, Wu S, et al. Breast cancer resistance to cyclin-dependent kinases 4/6 inhibitors: intricacy of the molecular mechanisms. Front Oncol. 2021;11:651541.
Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–39.
Demichele A, Clark AS, Tan KS, et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res. 2015;21(5):995–1001.
Fang H, Huang D, Yang F, et al. Potential biomarkers of CDK4/6 inhibitors in hormone receptor-positive advanced breast cancer. Breast Cancer Res Treat. 2018;168(2):287–97.
Currin E, Peterson LM, Schubert EK, et al. Temporal heterogeneity of estrogen receptor expression in bone-dominant breast cancer: 18F-fluoroestradiol PET imaging shows return of ER expression. J Natl Compr Canc Netw. 2016;14(2):144–7.
Schrijver W, Suijkerbuijk KPM, Van Gils CH, et al. Receptor conversion in distant breast cancer metastases: a systematic review and meta-analysis. J Natl Cancer Inst. 2018;110(6):568–80.
Aurilio G, Disalvatore D, Pruneri G, et al. A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur J Cancer. 2014;50(2):277–89.
Van Es SC, Van Der Vegt B, Bensch F, et al. Decalcification of breast cancer bone metastases with EDTA does not affect ER, PR, and HER2 results. Am J Surg Pathol. 2019;43(10):1355–60.
Liao GJ, Clark AS, Schubert EK, et al. 18F-fluoroestradiol PET: current status and potential future clinical applications. J Nucl Med. 2016;57(8):1269–75.
Hu X, Chen W, Li F, et al. Expression changes of ER, PR, HER2, and Ki-67 in primary and metastatic breast cancer and its clinical significance. Front Oncol. 2023;13:1053125.
Liu C, Gong C, Liu S, et al. (18)F-FES PET/CT influences the staging and management of patients with newly diagnosed estrogen receptor-positive breast cancer: a retrospective comparative study with (18)F-FDG PET/CT. Oncologist. 2019;24(12):e1277–85.
Van Kruchten M, De Vries EGE, Brown M, et al. PET imaging of oestrogen receptors in patients with breast cancer. Lancet Oncol. 2013;14(11):e465–75.
Yang Z, Sun Y, Xu X, et al. The assessment of estrogen receptor status and its intratumoral heterogeneity in patients with breast cancer by using 18F-fluoroestradiol PET/CT. Clin Nucl Med. 2017;42(6):421–7.
Kurland BF, Peterson LM, Lee JH, et al. Estrogen receptor binding (18F-FES PET) and glycolytic activity (18F-FDG PET) predict progression-free survival on endocrine therapy in patients with ER+ breast cancer. Clin Cancer Res. 2017;23(2):407–15.
Iqbal R, Yaqub M, Bektas HO, et al. [18F]FDG and [18F]FES PET/CT imaging as a biomarker for therapy effect in patients with metastatic ER+ breast cancer undergoing treatment with rintodestrant. Clin Cancer Res. 2023;29(11):2075–84.
Bottoni G, Piccardo A, Fiz F, et al. Heterogeneity of bone metastases as an important prognostic factor in patients affected by oestrogen receptor-positive breast cancer. The role of combined [18F]Fluoroestradiol PET/CT and [18F]Fluorodeoxyglucose PET/CT. Eur J Radiol. 2021;141:10821.
Yamada S, Tsuyoshi H, Yamamoto M, et al. Prognostic value of 16α-(18)F-fluoro-17β-Estradiol PET as a predictor of disease outcome in endometrial cancer: a prospective study. J Nucl Med. 2021;62(5):636–42.
Taralli S, Lorusso M, Scolozzi V, et al. Response evaluation with (18)F-FDG PET/CT in metastatic breast cancer patients treated with Palbociclib: first experience in clinical practice. Ann Nucl Med. 2019;33(3):193–200.
Ozawa H, Higuchi T, Fujimoto Y, et al. Total lesion glycolysis levels as predictive indicators in patients with metastatic and recurrent breast cancer undergoing endocrine therapy with or without CDK4/6 inhibitor. Anticancer Res. 2022;42(10):4813–24.
Seifert R, Küper A, Tewes M, et al. [18F]-fluorodeoxyglucose positron emission tomography/CT to assess the early metabolic response in patients with hormone receptor-positive HER2-negative metastasized breast cancer treated with cyclin-dependent 4/6 kinase inhibitors. Oncol Res Treat. 2021;44(7–8):400–7.
Boers J, Venema CM, De Vries EFJ, et al. Molecular imaging to identify patients with metastatic breast cancer who benefit from endocrine treatment combined with cyclin-dependent kinase inhibition. Eur J Cancer. 2020;126:11–20.
Liu C, Hu S, Xu X, et al. Evaluation of tumour heterogeneity by (18)F-fluoroestradiol PET as a predictive measure in breast cancer patients receiving palbociclib combined with endocrine treatment. Breast Cancer Res. 2022;24(1):57.
Boellaard R, Delgado-Bolton R, Oyen WJ, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–54.
Nienhuis HH, Van Kruchten M, Elias SG, et al. (18)F-fluoroestradiol tumor uptake is heterogeneous and influenced by site of metastasis in breast cancer patients. J Nucl Med. 2018;59(8):1212–8.
Zhao Z, Yoshida Y, Kurokawa T, et al. 18F-FES and 18F-FDG PET for differential diagnosis and quantitative evaluation of mesenchymal uterine tumors: correlation with immunohistochemical analysis. J Nucl Med. 2013;54(4):499–506.
Yamamoto M, Tsujikawa T, Yamada S, et al. 18F-FDG/18F-FES standardized uptake value ratio determined using PET predicts prognosis in uterine sarcoma. Oncotarget. 2017;8(14):22581–9.
Bottoni G, Fiz F, Puntoni M, et al. Diagnostic effectiveness of [(18)F]Fluoroestradiol PET/CT in oestrogen receptor-positive breast cancer: the key role of histopathology. Evidence from an international multicentre prospective study. Eur J Nucl Med Mol Imaging. 2023;50(8):2477–85.
Ulaner GA, Mankoff DA, Clark AS, et al. Summary: appropriate use criteria for estrogen receptor-targeted PET imaging with 16α-(18)F-fluoro-17β-fluoroestradiol. J Nucl Med. 2023;64(3):351–4.
Elmi A, Makvandi M, Weng CC, et al. Cell-proliferation imaging for monitoring response to CDK4/6 inhibition combined with endocrine-therapy in breast cancer: comparison of [(18)F]FLT and [(18)F]ISO-1 PET/CT. Clin Cancer Res. 2019;25(10):3063–73.
Franco J, Balaji U, Freinkman E, et al. Metabolic reprogramming of pancreatic cancer mediated by CDK4/6 inhibition elicits unique vulnerabilities. Cell Rep. 2016;14(5):979–90.
Van Geel JJL, De Vries EFJ, Van Kruchten M, et al. Molecular imaging as biomarker for treatment response and outcome in breast cancer. Ther Adv Med Oncol. 2023;15:17588359231170738.
Boers J, De Vries EFJ, Glaudemans A, et al. Application of PET tracers in molecular imaging for breast cancer. Curr Oncol Rep. 2020;22(8):85.
Van Kruchten M, Glaudemans AW, De Vries EF, et al. PET imaging of estrogen receptors as a diagnostic tool for breast cancer patients presenting with a clinical dilemma. J Nucl Med. 2012;53(2):182–90.
Van Kruchten M, Glaudemans A, De Vries EFJ, et al. Positron emission tomography of tumour [(18)F]fluoroestradiol uptake in patients with acquired hormone-resistant metastatic breast cancer prior to oestradiol therapy. Eur J Nucl Med Mol Imaging. 2015;42(11):1674–81.
Van Kruchten M, De Vries EG, Glaudemans AW, et al. Measuring residual estrogen receptor availability during fulvestrant therapy in patients with metastatic breast cancer. Cancer Discov. 2015;5(1):72–81.
Liu C, Xu X, Yuan H, et al. Dual tracers of 16α-[18F]fluoro-17β-Estradiol and [18F]fluorodeoxyglucose for prediction of progression-free survival after fulvestrant therapy in patients with HR+/HER2− metastatic breast cancer. Front Oncol. 2020;10:580277.
He M, Liu C, Shi Q, et al. The predictive value of early changes in (18) F-fluoroestradiol positron emission tomography/computed tomography during fulvestrant 500 mg therapy in patients with estrogen receptor-positive metastatic breast cancer. Oncologist. 2020;25(11):927–36.
Li Y, Liu C, Wang B, et al. Prediction of pretreatment 18F-FDG-PET/CT parameters on the outcome of first-line therapy in patients with metastatic breast cancer. Int J Gen Med. 2021;14:1797–809.
Diao W, Tian F, Jia Z. The prognostic value of SUV(max) measuring on primary lesion and ALN by (18)F-FDG PET or PET/CT in patients with breast cancer. Eur J Radiol. 2018;105:1–7.
Anurag M, Haricharan S, Ellis MJ. CDK4/6 inhibitor biomarker research: Are we barking up the wrong tree? Clin Cancer Res. 2020;26(1):3–5.
Lorito N, Bacci M, Smiriglia A, et al. Glucose metabolic reprogramming of ER breast cancer in acquired resistance to the CDK4/6 inhibitor palbociclib. Cells. 2020;9(3):1.
O’mahony F, Razandi M, Pedram A, et al. Estrogen modulates metabolic pathway adaptation to available glucose in breast cancer cells. Mol Endocrinol. 2012;26(12):2058–70.
Funding
This work was supported by grants from the Science and Technology Development Fund of Shanghai Pudong New Area (PKJ2020-Y54), Shanghai Sailing Program (20YF1408500) and Shanghai Committee of Science and Technology Fund (22DZ2204500).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, C., Ma, G., Zhang, J. et al. 18F-FES and 18F-FDG PET/CT imaging as a predictive biomarkers for metastatic breast cancer patients undergoing cyclin-dependent 4/6 kinase inhibitors with endocrine treatment. Ann Nucl Med 37, 675–684 (2023). https://doi.org/10.1007/s12149-023-01871-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-023-01871-8