Abstract
Background
For differentiated thyroid cancer (DTC) patients with thyroglobulin (Tg) elevation and negative iodine scintigraphy (commonly termed "TENIS" syndrome) after thyroidectomy, radioactive iodine (RAI) therapy, and thyroid-stimulating hormone (TSH) suppression therapy, empirical RAI therapy may be considered. However, the outcome data of TENIS syndrome without structural disease after empirical RAI therapy have not shown clear evidence of improvement in survival. We assessed the efficacy of such empirical RAI therapy in TENIS syndrome without structural disease and evaluated the progression-free survival (PFS).
Methods
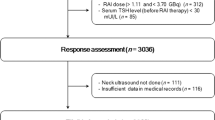
A total of 80 papillary thyroid cancer (PTC) patients with TENIS syndrome without structural disease were included in this retrospective study. 52 patients were treated with empirical RAI therapy while another 28 patients were untreated. The progression-free survival (PFS) of both groups was defined as the main outcome. The secondary outcome was the comparison of serum Tg levels 12 months after being diagnosed as TENIS syndrome.
Results
The PFS of the empirical RAI therapy group was better than the untreated group (p < 0.001). Moreover, there was significant difference in Tg normalization between patients treated with empirical therapy and without treatment (p = 0.001). Empirical RAI therapy (p = 0.001) predicts better PFS. Male gender (p = 0.041) and empirical RAI therapy (p = 0.002) predict better remission in serum Tg level.
Conclusion
Patients with TENIS syndrome without structural disease can benefit from empirical RAI therapy in both PFS and Tg normalization.


Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Klain M, Pace L, Zampella E, et al. Outcome of patients with differentiated thyroid cancer treated with 131-iodine on the basis of a detectable serum thyroglobulin level after initial treatment. Front Endocrinol (Lausanne). 2019;10:146.
Basu S, Dandekar M, Joshi A, et al. Defining a rational step-care algorithm for managing thyroid carcinoma patients with elevated thyroglobulin and negative on radioiodine scintigraphy (TENIS): considerations and challenges towards developing an appropriate roadmap. Eur J Nucl Med Mol Imaging. 2015;42:1167–71.
Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133.
Kabasakal L, Selcuk NA, Shafipour H, et al. Treatment of iodine-negative thyroglobulin-positive thyroid cancer: differences in outcome in patients with macrometastases and patients with micrometastases. Eur J Nucl Med Mol Imaging. 2004;31:1500–4.
Sabra MM, Grewal RK, Tala H, et al. Clinical outcomes following empiric radioiodine therapy in patients with structurally identifiable metastatic follicular cell-derived thyroid carcinoma with negative diagnostic but positive post-therapy 131I whole-body scans. Thyroid. 2012;22:877–83.
Tramontin MY, Nobre GM, Lopes M, et al. High thyroglobulin and negative whole-body scan: no long-term benefit of empiric radioiodine therapy. Endocrine. 2021;73:398–406.
Gianoukakis AG. Thyroglobulin antibody status and differentiated thyroid cancer: what does it mean for prognosis and surveillance? Curr Opin Oncol. 2015;27:26–32.
Suteau V, Munier M, Briet C, et al. Sex bias in differentiated thyroid cancer. Int J Mol Sci. 2021;22(23):12992.
Preissner CM, O’Kane DJ, Singh RJ, et al. Phantoms in the assay tube: heterophile antibody interferences in serum thyroglobulin assays. J Clin Endocrinol Metab. 2003;88:3069–74.
Cheng L, Sa R, Luo Q, et al. Unexplained hyperthyroglobulinemia in differentiated thyroid cancer patients as an indication for radioiodine adjuvant therapy: a prospective multicenter study. J Nucl Med. 2021;62:62–8.
Shinohara S, Kikuchi M, Suehiro A, et al. Characteristics and prognosis of patients with thyroglobulin-positive and radioactive iodine whole-body scan-negative differentiated thyroid carcinoma. Jpn J Clin Oncol. 2015;45:427–32.
Kim WG, Ryu JS, Kim EY, et al. Empiric high-dose 131-iodine therapy lacks efficacy for treated papillary thyroid cancer patients with detectable serum thyroglobulin, but negative cervical sonography and 18F-fluorodeoxyglucose positron emission tomography scan. J Clin Endocrinol Metab. 2010;95:1169–73.
Carrillo JF, Vazquez-Romo R, Ramirez-Ortega MC, et al. Prognostic impact of direct (131)I therapy after detection of biochemical recurrence in intermediate or high-risk differentiated thyroid cancer: a retrospective cohort study. Front Endocrinol (Lausanne). 2019;10:737.
Rosario PW, Mourao GF, dos Santos JB, et al. Is empirical radioactive iodine therapy still a valid approach to patients with thyroid cancer and elevated thyroglobulin? Thyroid. 2014;24:533–6.
Fatourechi V, Hay ID, Javedan H, et al. Lack of impact of radioiodine therapy in tg-positive, diagnostic whole-body scan-negative patients with follicular cell-derived thyroid cancer. J Clin Endocrinol Metab. 2002;87:1521–6.
Wang H, Dai H, Li Q, et al. Investigating (18)F-FDG PET/CT parameters as prognostic markers for differentiated thyroid cancer: a systematic review. Front Oncol. 2021;11: 648658.
Liu M, Cheng L, Jin Y, et al. Predicting I-avidity of metastases from differentiated thyroid cancer using F-FDG PET/CT in postoperative patients with elevated thyroglobulin. Sci Rep. 2018;8:4352.
Salvatore B, Klain M, Nicolai E, et al. Prognostic role of FDG PET/CT in patients with differentiated thyroid cancer treated with 131-iodine empiric therapy. Medicine (Baltimore). 2017;96: e8344.
Stangierski A, Kaznowski J, Wolinski K, et al. The usefulness of fluorine-18 fluorodeoxyglucose PET in the detection of recurrence in patients with differentiated thyroid cancer with elevated thyroglobulin and negative radioiodine whole-body scan. Nucl Med Commun. 2016;37:935–8.
Acknowledgements
The authors are grateful to Zekun Tan for his help with the assistance of statistics in this paper.
Author information
Authors and Affiliations
Contributions
Conceptualization: LY, HF, LP, WO. Methodology: LY, LP, JL, WO. Formal analysis and investigation: LY, JX, XX, JW. Writing-original draft preparation: LY, JW, JX, PC. Writing-review and editing: LY, JW, JL, WO. Supervision: LY, JW, HF, WO.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, L., Wang, J., Pan, L. et al. Outcome of patients with differentiated thyroid cancer treated with empirical radioiodine therapy on the basis of Thyroglobulin Elevation Negative Iodine Scintigraphy (TENIS) syndrome without structural disease: a retrospective cohort study. Ann Nucl Med 37, 18–25 (2023). https://doi.org/10.1007/s12149-022-01799-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-022-01799-5