Abstract
Purpose
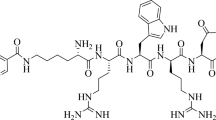
In this study, we designed a new linear 6-Hydrazinonicotinamide (HYNIC)-conjugated peptide (HYNIC-KRWrNM) (M-6) and labeled with technetium-99m for gamma imaging of glioblastoma as a αvβ3-positive tumor. We evaluated tumor targeting ability of this radio-peptide and compared with previous 99mTc-labeled HYNIC-conjugated RGD analogue peptides.
Procedures.
One new linear peptide (HYNIC-KRWrNM) (M-6) was designed and labeled with technetium-99m in the presence of 2-[[1,3-dihydroxy-2-(hydroxymethyl) propan-2-yl] amino] acetic acid (Tricine)/Ethylenediamine-N,N′-diacetic acid (EDDA) as co-ligand system. Then, this 99mTc-labeled peptide ([99mTc]Tc-M-7) was evaluated for in vitro stability in saline and serum, specific binding assay, internalization, and binding affinity (Kd). In addition, we performed biodistribution study and planar imaging on nude mice bearing U87-MG xenograft as a αvβ3-positive tumor.
Results
The radiochemical yield of [99mTc]Tc-M-7 was obtained ˃95%. This 99mTc-labeled peptide remained stable and intact in saline solution after 24 h incubation. In addition, metabolic stability of this 99mTc-labeled peptide was obtained ˃60% after 4 h incubation in serum. The Kd value for [99mTc]Tc-M-7 was obtained 5.2 ± 1.0 nM. Based on biodistribution results in nude mice bearing U87-MG xenograft, tumor/muscle activity ratio was 6.22 and decreased to 1.89 in blocking group at the same time point (4 h p.i.). The blocking experiment results also indicated that tumor uptake and kidney uptake were αvβ3-mediated. In comparison with previous HYNIC-conjugated RGD analogue peptides, kidneys had the highest uptake of this 99mTc-labeled peptide (52.29 ± 11.48 at 1.5 h p.i. and 27.04 ± 0.66%ID/g at 4 h p.i.). Finally, similar to previous 99mTc-labeled HYNIC-conjugated RGD analogue peptides, [99mTc]Tc-M-7 showed acceptable tumor uptake after 4 h post-injection (based on ROI technique, target-to-background activity ratio = 3.80).
Conclusions
This small linear 99mTc-labeled peptide, with high affinity to αvβ3 integrin, desirable water solubility, and cost efficient, demonstrates a potent tumor targeting ability as well as previous HYNIC-conjugated RGD analogue peptides. Hence, [99mTc]Tc-M-7 can be of service to as a new candidate for early detection of αvβ3-positive tumors.












Similar content being viewed by others
Change history
12 October 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12149-022-01793-x
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. Cancer J Clin. 2011;61:69–90.
Shah SC, Kayamba V, Peek RM Jr, Heimburger D. Cancer control in low-and middle-income countries: is it time to consider screening? J Global Oncol. 2019;5:1–8.
Weir HK, Thompson TD, Soman A, Møller B, Leadbetter S. The past, present, and future of cancer incidence in the United States: 1975 through 2020. Cancer. 2015;121:1827–37.
Weissleder R. Molecular imaging in cancer. Science. 2006;312:1168–71.
Basit R, Singh SK. Applications of molecular imaging techniques in oncology. In: Shashank KS, Reena S, Chirag C, editors. Modern cancer therapies and traditional medicine: an integrative approach to combat cancers. Netherlands: Bentham Science Publishers; 2021.
Chen Z-Y, Wang Y-X, Lin Y, et al. Advance of molecular imaging technology and targeted imaging agent in imaging and therapy. BioMed Res Int. 2014;2014:819324.
Maman S, Witz IP. A history of exploring cancer in context. Nat Rev Cancer. 2018;18:359–76.
Lopes-Bastos BM, Jiang WG, Cai J. Tumour–endothelial cell communications: important and indispensable mediators of tumour angiogenesis. Anticancer Res. 2016;36:1119–26.
Kaihani S, Sadeghzadeh N. Study of the 99mTc-labeling conditions of 6-hydrazinonicotinamide-conjugated peptides from a new perspective: Introduction to the term radio-stoichiometry. J Labelled Compd Radiopharm. 2020;63:582–96.
Debordeaux F, Chansel-Debordeaux L, Pinaquy J-B, Fernandez P, Schulz J. What about αvβ3 integrins in molecular imaging in oncology? Nucl Med Biol. 2018;62:31–46.
Schittenhelm J, Tabatabai G, Sipos B. The role of integrins in primary and secondary brain tumors. Histol Histopathol. 2016;31:1069–78.
Kapp TG, Rechenmacher F, Neubauer S, et al. A comprehensive evaluation of the activity and selectivity profile of ligands for RGD-binding integrins. Sci Rep. 2017;7:1–13.
Wu P-H, Opadele AE, Onodera Y, Nam J-M. Targeting integrins in cancer nanomedicine: applications in cancer diagnosis and therapy. Cancers. 2019;11:1783.
Ma Y, Ai G, Zhang C, et al. Novel linear peptides with high affinity to αvβ3 integrin for precise tumor identification. Theranostics. 2017;7:1511.
Liu S. Radiolabeled cyclic RGD peptide bioconjugates as radiotracers targeting multiple integrins. Bioconjug Chem. 2015;26:1413–38.
Emrarian I, Sadeghzadeh N, Abedi SM, Abediankenari S. New neurotensin analogue radiolabeled by 99m-technetium as a potential agent for tumor identification. Chem Biol Drug Des. 2018;91:304–13.
Meszaros LK, Dose A, Biagini SC, Blower PJ. Synthesis and evaluation of analogues of HYNIC as bifunctional chelators for technetium. Dalton Trans. 2011;40:6260–7.
King RC, Surfraz MB-U, Biagini SC, Blower PJ, Mather SJ. How do HYNIC-conjugated peptides bind technetium? Insights from LC–MS and stability studies. Dalton Trans. 2007;43:4998–5007.
Ranjbar L, Maleki F, Sadeghzadeh N, Abediankenari S, Mardanshahi A, Masteri Farahani A. In vitro/in vivo assessment of the targeting ability of [99mTc] Tc-labeled an aptide specific to the extra domain B of fibronectin (APTEDB) for colorectal cancer. Ann Nucl Med. 2020;34:460–6.
Danhier F, Le Breton A, Vr P. RGD-based strategies to target alpha (v) beta (3) integrin in cancer therapy and diagnosis. Mol Pharm. 2012;9:2961–73.
Tornesello AL, Buonaguro L, Tornesello ML, Buonaguro FM. New insights in the design of bioactive peptides and chelating agents for imaging and therapy in oncology. Molecules. 2017;22:1282.
Zhao J, Chen J, Ma S, et al. Recent developments in multimodality fluorescence imaging probes. Acta Pharm Sinica B. 2018;8:320–38.
Ranjita S. Nanosuspensions: a new approach for organ and cellular targeting in infectious diseases. J Pharm Investig. 2013;43:1–26.
Feni L, Parente S, Cm R, et al. Kiss and run: Promoting effective and targeted cellular uptake of a drug delivery vehicle composed of an integrin-targeting diketopiperazine peptidomimetic and a cell-penetrating peptide. Bioconjug Chem. 2019;30:2011–22.
Huang C, Zhao X, Su M, Yin Z. Construction and evaluation of novel αvβ3 integrin ligand-conjugated ultrasmall star polymer micelles targeted glomerular podocytes through GFB permeation. Biomaterials. 2021;276: 121053.
Chigoho DM, Bridoux J, Hernot S. Reducing the renal retention of low-to moderate-molecular-weight radiopharmaceuticals. Curr Opin Chem Biol. 2021;63:219–28.
Satpati D, Vats K, Sharma R, Sarma HD, Dash A. 68Ga-labeling of internalizing RGD (iRGD) peptide functionalized with DOTAGA and NODAGA chelators. J Pept Sci. 2020;26:e3241.
Decristoforo C, Faintuch-Linkowski B, Rey A, et al. [99mTc] HYNIC-RGD for imaging integrin αvβ3 expression. Nucl Med Biol. 2006;33:945–52.
Ji S, Czerwinski A, Zhou Y, et al. 99mTc-Galacto-RGD2: a novel 99mTc-labeled cyclic RGD peptide dimer useful for tumor imaging. Mol Pharm. 2013;10:3304–14.
Karimi H, Sadeghzadeh N, Abediankenari S, Rezazadeh F, Hallajian F. Radiochemical evaluation and in vitro assessment of the targeting ability of a novel 99mTc-HYNIC-RGD for U87MG human brain cancer cells. Curr Radiopharm. 2017;10:139–44.
Ramezanizadeh M, Masterifarahani A, Sadeghzadeh N, Abediankenari S, Mardanshahi A, Maleki F. 99mTc-D (RGD): molecular imaging probe for diagnosis of αvβ3-positive tumors. Nucl Med Commun. 2020;41:104–9.
Jia B, Shi J, Yang Z, Xu B, Liu Z, Zhao H, et al. 99mTc-labeled cyclic RGDfK dimer: initial evaluation for SPECT imaging of glioma integrin αvβ3 expression. Bioconjug Chem. 2006;17:1069–76.
Wang J, Kim Y-S, Liu S. 99mTc-labeling of HYNIC-conjugated cyclic RGDfK dimer and tetramer using EDDA as coligand. Bioconjug Chem. 2008;19:634–42.
Bohn P, Modzelewski R, Rouvet J, Briand M, Dutoit S, Pille JY, et al. Biodistribution and imaging of [99mtc]-HYNIC-RGD in MDA-MB-231 and NTERA-2 cancer cell xenografts. Nucl Med Commun. 2013;34:709–17.
Shi J, Wang L, Kim YS, et al. Improving tumor uptake and excretion kinetics of 99mTc-labeled cyclic arginine-glycine-aspartic (RGD) dimers with triglycine linkers. J Med Chem. 2008;51:7980–90.
Acknowledgements
This work was the subject part of the thesis of Sajad Kaihani as a PhD student of the Mazandaran University of Medical Sciences and was supported with grant number 6301.
Funding
This study was supported by Mazandaran University of Medical Sciences, 6301, Nourollah Sadeghzadeh.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaihani, S., Sadeghzadeh, N., Abediankenari, S. et al. [99mTc]-labeling and evaluation of a new linear peptide for imaging of glioblastoma as a αvβ3-positive tumor. Ann Nucl Med 36, 976–985 (2022). https://doi.org/10.1007/s12149-022-01786-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-022-01786-w