Abstract
Objective
We previously reported that alterations of the tumor microenvironment (TME) by programmed death receptor-1 (PD1) blockade affected tumor glucose metabolism and tumor 2-deoxy-2-[18F]fluoro-D-glucose ([18F]FDG) uptake. In cancer cells, high glycolysis allows cells to sustain rapid proliferation since glycolysis is closely related to the proliferation of cancer cells. Therefore, imaging of cellular proliferation may provide more detail of TME alterations. In this study, we investigated how TME alterations by PD1 blockade affects the uptake of 3′-deoxy-3′-[18F]fluorothymidine ([18F]FLT), which is a 18F-radiolabeled thymidine derivative and is taken up by proliferating cells.
Methods
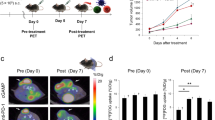
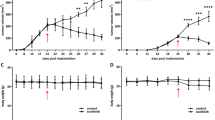
Mice inoculated with murine colon carcinoma CT26 cells were intraperitoneally administered an anti-PD1 antibody on Day 0, when the tumor volume exceeded 50 mm3, and Day 5. [18F]FLT-PET imaging was performed pre-treatment (Day 0) and post treatment (Day 7). Tumor infiltrating lymphocytes (TILs) were identified by flow cytometry. [18F]FLT accumulation and localization in tumor tissue was evaluated by autoradiography and immunohistochemistry. The cell-cycle distribution of tumors and CT26 cells exposed to cytokines (interleukin-2, interferon [INF]-γ, and tumor necrosis factor [TNF]-α) was analyzed by flow cytometry.
Results
PD1 blockade increased CD8+ and CD4+ T cells in tumor tissue and significantly suppressed tumor proliferation; however, tumor [18F]FLT uptake remained unchanged. Autoradiography and immunohistochemistry showed that [18F]FLT was mainly taken up by cancer cells, but not TILs. Flow cytometric analysis demonstrated that the population of cells in G2/M phase increased after PD1 blockade. Moreover, INF-γ and TNF-α significantly increased cells in G2/M phase in vitro.
Conclusion
PD1 blockade-induced alteration of the TME increased CT26 tumor cells in the G2/M phase, which have high thymidine kinase 1 activity. Therefore, [18F]FLT is taken up by tumor cells even if tumor proliferation is suppressed. This observation may be useful for evaluating the response to immunotherapy.





Similar content being viewed by others
Abbreviations
- [18F]FDG:
-
2-Deoxy-2-[18F]fluoro-D-glucose
- [18F]FLT:
-
3′-Deoxy-3′-[18F]fluorothymidine
- IFN-γ:
-
Interferon gamma; IL-2, interleukin-2
- PET:
-
Positron emission tomography
- PD1:
-
Programmed cell death-1
- RT:
-
Room temperature
- TME:
-
Tumor-microenvironment
- TIL:
-
Tumor infiltrating lymphocyte
- TK1:
-
Thymidine kinase 1
- TNF-α:
-
Tumor necrosis factor alpha
References
Lipson EJ, Forde PM, Hammers HJ, Emens LA, Taube JM, Topalian SL. Antagonists of PD-1 and PD-L1 in cancer treatment. Semin Oncol. 2015;42:587–600.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20.
Unterrainer M, Ruzicka M, Fabritius MP, Mittlmeier LM, Winkelmann M, Rubenthaler J, et al. PET/CT imaging for tumor response assessment to immunotherapy: current status and future directions. Eur Radiol Exp. 2020;4:63.
Dromain C, Beigelman C, Pozzessere C, Duran R, Digklia A. Imaging of tumor response to immunotherapy. Eur Radiol Exp. 2020;4:2.
Bollineni VR, Collette S, Liu Y. Functional and molecular imaging in cancer drug development. Chin Clin Oncol. 2014;3:17.
Tomita M, Yasui H, Higashikawa K, Nakajima K, Takakura H, Shiga T, et al. Anti PD-1 treatment increases [(18)F]FDG uptake by cancer cells in a mouse B16F10 melanoma model. EJNMMI Res. 2018;8:82.
Tomita M, Suzuki M, Kono Y, Nakajima K, Matsuda T, Kuge Y, et al. Influence on [(18)F]FDG uptake by cancer cells after anti-PD-1 therapy in an enforced-immune activated mouse tumor. EJNMMI Res. 2020;10:24.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33.
Shields AF, Grierson JR, Dohmen BM, Machulla HJ, Stayanoff JC, Lawhorn-Crews JM, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998;4:1334–6.
Bollineni VR, Kramer GM, Jansma EP, Liu Y, Oyen WJ. A systematic review on [(18)F]FLT-PET uptake as a measure of treatment response in cancer patients. Eur J Cancer. 2016;55:81–97.
Everitt SJ, Ball DL, Hicks RJ, Callahan J, Plumridge N, Collins M, et al. Differential (18)F-FDG and (18)F-FLT Uptake on Serial PET/CT imaging before and during definitive chemoradiation for non-small cell lung cancer. J Nucl Med. 2014;55:1069–74.
Yun M, Oh SJ, Ha HJ, Ryu JS, Moon DH. High radiochemical yield synthesis of 3’-deoxy-3’-[18F]fluorothymidine using (5’-O-dimethoxytrityl-2’-deoxy-3’-O-nosyl-beta-D-threo pentofuranosyl)thymine and its 3-N-BOC-protected analogue as a labeling precursor. Nucl Med Biol. 2003;30:151–7.
Rasey JS, Grierson JR, Wiens LW, Kolb PD, Schwartz JL. Validation of FLT uptake as a measure of thymidine kinase-1 activity in A549 carcinoma cells. J Nucl Med. 2002;43:1210–7.
Lai WY, Huang BT, Wang JW, Lin PY, Yang PC. A novel PD-L1-targeting antagonistic DNA aptamer with antitumor effects. Mol Ther Nucleic Acids. 2016;5: e397.
Aarntzen EH, Srinivas M, De Wilt JH, Jacobs JF, Lesterhuis WJ, Windhorst AD, et al. Early identification of antigen-specific immune responses in vivo by [18F]-labeled 3’-fluoro-3’-deoxy-thymidine ([18F]FLT) PET imaging. Proc Natl Acad Sci U S A. 2011;108:18396–9.
Ribas A, Benz MR, Allen-Auerbach MS, Radu C, Chmielowski B, Seja E, et al. Imaging of CTLA4 blockade-induced cell replication with (18)F-FLT PET in patients with advanced melanoma treated with tremelimumab. J Nucl Med. 2010;51:340–6.
Sherley JL, Kelly TJ. Regulation of human thymidine kinase during the cell cycle. J Biol Chem. 1988;263:8350–8.
Roppongi M, Izumisawa M, Terasaki K, Muraki Y, Shozushima M. (18)F-FDG and (11)C-choline uptake in proliferating tumor cells is dependent on the cell cycle in vitro. Ann Nucl Med. 2019;33:237–43.
Sung Y, Tetrault MA, Takahashi K, Ouyang J, Pratx G, Fakhri GE, et al. Dependence of fluorodeoxyglucose (FDG) uptake on cell cycle and dry mass: a single-cell study using a multi-modal radiography platform. Sci Rep. 2020;10:4280.
Reichert TE, Nagashima S, Kashii Y, Stanson J, Gao G, Dou QP, et al. Interleukin-2 expression in human carcinoma cell lines and its role in cell cycle progression. Oncogene. 2000;19:514–25.
Burke F, East N, Upton C, Patel K, Balkwill FR. Interferon gamma induces cell cycle arrest and apoptosis in a model of ovarian cancer: enhancement of effect by batimastat. Eur J Cancer. 1997;33:1114–21.
Faurschou A, Gniadecki R, Calay D, Wulf HC. TNF-alpha impairs the S-G2/M cell cycle checkpoint and cyclobutane pyrimidine dimer repair in premalignant skin cells: role of the PI3K-Akt pathway. J Invest Dermatol. 2008;128:2069–77.
Acknowledgements
This work was supported, in part, by JST CREST Grant Number JPMJCR1902; JSPS KAKENHI Grant Number 19H03593, 20K16813; AMED Grant Number JP21zf0127004; the Photo-excitonix Project in Hokkaido University; Takeda Science Foundation. We would like to thank the staff of the Hokkaido University Hospital Cyclotron Facility for synthesis of [18F]FLT.
Funding
Core Research for Evolutional Science and Technology, JPMJCR1902, Mikako Ogawa, Japan Society for the Promotion of Science, 19H03593, Mikako Ogawa, 20K16813, Motofumi Suzuki, Takeda Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Suzuki, M., Matsuda, T., Nakajima, K. et al. PD1 blockade alters cell-cycle distribution and affects 3′-deoxy-3′-[18F]fluorothymidine uptake in a mouse CT26 tumor model. Ann Nucl Med 36, 931–940 (2022). https://doi.org/10.1007/s12149-022-01782-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-022-01782-0