Abstract
Objective
The aim of this study was to evaluate the radiation dosimetry of alpha-emitter 225Ac-DOTA-rituximab using Monte Carlo simulation of 64Cu-DOTA-rituximab.
Methods
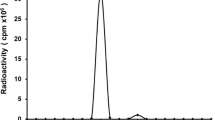
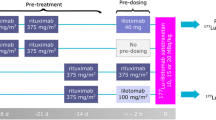
CD20 expression was evaluated in lymphoma cell lines (Jurkat and Raji). DOTA-rituximab was conjugated and then chelated by 64Cu. Tumor xenograft models were established in BALB/c-nu mice. Animal PET/CT imaging was obtained after tail vein injection with and without a pre-dose of 2 mg of cold rituximab. Specific binding of tumors was evaluated by an organ distribution assay and autoradiography. CD20 expression in tumor tissues was evaluated by immunohistochemistry. The residence time was calculated using 64Cu-DOTA-rituximab PET/CT acquisition data using OLINDA/EXM software. 225Ac-DOTA-rituximab tumor dosimetry was performed using Monte Carlo simulation with 64Cu-DOTA-rituximab PET/CT images.
Results
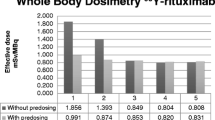
Specific binding of Raji cells (CD20 positive) was 90 times that of Jurkat cells (CD20 negative) (p < 0.0001). Immunoreactivity was more than 75%. PET/CT imaging with 64Cu-DOTA-rituximab was specifically observed in tumors. The radioactivity of the tumor was much higher than that of other organs, and tumor uptake was related to CD20 expression. The predicted human dose for the administration of 64Cu-DOTA-rituximab was measured as the effective dose (1.07E-02 mSv/MBq). In the tumor region, equivalent doses of 225Ac-DOTA-rituximab (14 SvRBE5/MBq) were much higher (74-fold) than those of 64Cu-DOTA-rituximab (0.19 SvRBE5/MBq) (p < 0.01).
Conclusion
Tumor dosimetry of 225Ac-DOTA-rituximab can be estimated via the Monte Carlo simulation of 64Cu-DOTA-rituximab. 225Ac-DOTA-rituximab can be employed for lymphoma as targeted alpha therapy.



Similar content being viewed by others
References
Hagenbeek A. Radioimmunotherapy for NHL: experience of 90Y-ibritumomab tiuxetan in clinical practice. Leuk Lymphoma. 2003;44(Suppl 4):S37-47.
Kaminski MS, Estes J, Zasadny KR, et al. Radioimmunotherapy with iodine (131)I tositumomab for relapsed or refractory B-cell non-Hodgkin lymphoma: updated results and long-term follow-up of the University of Michigan experience. Blood. 2000;96:1259–66.
Kaminski MS, Tuck M, Estes J, et al. 131I-tositumomab therapy as initial treatment for follicular lymphoma. N Engl J Med. 2005;352:441–9.
Bienert M, Reisinger I, Srock S, et al. Radioimmunotherapy using 131I-rituximab in patients with advanced stage B-cell non-Hodgkin’s lymphoma: initial experience. Eur J Nucl Med Mol Imaging. 2005;32:1225–33.
Leahy MF, Seymour JF, Hicks RJ, et al. Multicenter phase II clinical study of iodine-131-rituximab radioimmunotherapy in relapsed or refractory indolent non-Hodgkin’s lymphoma. J Clin Oncol. 2006;24:4418–25.
Turner JH. Defining pharmacokinetics for individual patient dosimetry in routine radiopeptide and radioimmunotherapy of cancer: Australian experience. Curr Pharm Des. 2009;15:966–82.
Kang HJ, Lee SS, Byun BH, et al. Repeated radioimmunotherapy with 131I-rituximab for patients with low-grade and aggressive relapsed or refractory B cell non-Hodgkin lymphoma. Cancer Chemother Pharmacol. 2013;71:945–53.
Kang GW, Kang HJ, Shin DY, et al. Radioimmunotherapy with (131)i-rituximab in a patient with diffuse large B-cell lymphoma relapsed after treatment with (90)y-ibritumomab tiuxetan. Nucl Med Mol Imaging. 2013;47:281–4.
Kang HJ, Lee SS, Kim KM, et al. Radioimmunotherapy with (131)I-rituximab for patients with relapsed/refractory B-cell non-Hodgkin’s lymphoma (NHL). Asia Pac J Clin Oncol. 2011;7:136–45.
Lee I, Byun BH, Lim I, et al. Comparisons of 131I-rituximab treatment responses in patients with aggressive lymphoma and indolent lymphoma. Ann Nucl Med. 2019;33:881–90.
Lim I, Park JY, Kang HJ, et al. Prognostic significance of pretreatment 18F-FDG PET/CT in patients with relapsed/refractory B-cell non-Hodgkin’s lymphoma treated by radioimmunotherapy using 131I-rituximab. Acta Haematol. 2013;130:74–82.
Shin DY, Byun BH, Kim KM, et al. Radioimmunotherapy with (131)I-rituximab as consolidation therapy for patients with diffuse large B-cell lymphoma. Cancer Chemother Pharmacol. 2016;78:825–31.
Seidl C. Radioimmunotherapy with α-particle-emitting radionuclides. Immunotherapy. 2014;6:431–58.
McDevitt MR, Sgouros G, Finn RD, et al. Radioimmunotherapy with alpha-emitting nuclides. Eur J Nucl Med. 1998;25:1341–51.
Essler M, Gärtner FC, Neff F, et al. Therapeutic efficacy and toxicity of 225Ac-labelled vs. 213Bi-labelled tumour-homing peptides in a preclinical mouse model of peritoneal carcinomatosis. Eur J Nucl Med Mol Imaging. 2012;39:602–12.
Kratochwil C, Bruchertseifer F, Giesel FL, et al. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J Nucl Med. 2016;57:1941–4.
Sgouros G, Roeske JC, McDevitt MR, et al. MIRD Pamphlet No. 22 (abridged): radiobiology and dosimetry of alpha-particle emitters for targeted radionuclide therapy. J Nucl Med. 2010;51:311–28.
Borchardt PE, Yuan RR, Miederer M, et al. Targeted actinium-225 in vivo generators for therapy of ovarian cancer. Cancer Res. 2003;63:5084–90.
Behling K, Maguire WF, López Puebla JC, et al. Vascular targeted radioimmunotherapy for the treatment of glioblastoma. J Nucl Med. 2016;57:1576–82.
Pommé S, Marouli M, Suliman G, et al. Measurement of the 225Ac half-life. Appl Radiat Isot. 2012;70:2608–14.
Reff ME, Carner K, Chambers KS, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–45.
Kim JY, Park H, Lee JC, et al. Simple Cu-64 production and its application of Cu-64 ATSM. Appl Radiat Isot. 2009;67:1190–4.
Lindmo T, Boven E, Cuttitta F, et al. Determination of the immunoreactive function of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods. 1984;72:77–89.
Snyder WS (1975) "S" absorbed dose per unit cumulated activity for selected radionuclides and organs. MIRD Pamphlet no. 11
Natarajan A, Gowrishankar G, Nielsen CH, et al. Positron emission tomography of 64Cu-DOTA-Rituximab in a transgenic mouse model expressing human CD20 for clinical translation to image NHL. Mol Imaging Biol. 2012;14:608–16.
Woo SK, Jang SJ, Seo MJ, et al. Development of 64Cu-NOTA-trastuzumab for HER2 targeting: a radiopharmaceutical with improved pharmacokinetics for human studies. J Nucl Med. 2019;60:26–33.
Dahle J, Borrebaek J, Jonasdottir TJ, et al. Targeted cancer therapy with a novel low-dose rate alpha-emitting radioimmunoconjugate. Blood. 2007;110:2049–56.
Dahle J, Borrebaek J, Melhus KB, et al. Initial evaluation of (227)Th-p-benzyl-DOTA-rituximab for low-dose rate alpha-particle radioimmunotherapy. Nucl Med Biol. 2006;33:271–9.
Dahle J, Bruland OS, Larsen RH. Relative biologic effects of low-dose-rate alpha-emitting 227Th-rituximab and beta-emitting 90Y-tiuexetan-ibritumomab versus external beam X-radiation. Int J Radiat Oncol Biol Phys. 2008;72:186–92.
McLaughlin P, Grillo-López AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–33.
Huang SY, Bolch WE, Lee C, et al. Patient-specific dosimetry using pretherapy [124I]m-iodobenzylguanidine ([124I]mIBG) dynamic PET/CT imaging before [131I]mIBG targeted radionuclide therapy for neuroblastoma. Mol Imaging Biol. 2015;17:284–94.
Kratochwil C, Schmidt K, Afshar-Oromieh A, et al. Targeted alpha therapy of mCRPC: dosimetry estimate of 213Bismuth-PSMA-617. Eur J Nucl Med Mol Imaging. 2018;45:31–7.
Wadas TJ, Wong EH, Weisman GR, et al. Copper chelation chemistry and its role in copper radiopharmaceuticals. Curr Pharm Des. 2007;13:3–16.
McDevitt MR, Ma D, Lai LT, et al. Tumor therapy with targeted atomic nanogenerators. Science. 2001;294:1537–40.
Sgouros G, Hobbs RF. Dosimetry for radiopharmaceutical therapy. Semin Nucl Med. 2014;44:172–8.
Couturier O, Supiot S, Degraef-Mougin M, et al. Cancer radioimmunotherapy with alpha-emitting nuclides. Eur J Nucl Med Mol Imaging. 2005;32:601–14.
Azorín-Vega E, Rojas-Calderón E, Ferro-Flores G, et al. Assessment of the radiation absorbed dose produced by 177Lu-iPSMA, 225Ac-iPSMA and 223RaCl2 to prostate cancer cell nuclei in a bone microenvironment model. Appl Radiat Isot. 2019;146:66–71.
Schwartz J, Jaggi J, O’Donoghue J, et al. Renal uptake of bismuth-213 and its contribution to kidney radiation dose following administration of actinium-225-labeled antibody. Phys Med Biol. 2011;56(3):721–33.
Paudyal P, Paudyal B, Hanaoka H, et al. Imaging and biodistribution of Her2/neu expression in non-small cell lung cancer xenografts with Cu-labeled trastuzumab PET. Cancer Sci. 2010;101:1045–50.
Li L, Rousseau J, Jaraquemada-Peláez MG, et al. 225Ac-H4py4pa for targeted alpha therapy. Bioconjug Chem. 2020. https://doi.org/10.1021/acs.bioconjchem.0c00171.
Watabe T, Liu Y, Kaneda-Nakashima K, et al. Theranostics targeting fibroblast activation protein in the tumor stroma: 64Cu- and 225Ac-labeled FAPI-04 in pancreatic cancer xenograft mouse models. J Nucl Med. 2020;61:563–9.
Acknowledgements
This study was supported by a Grant from the Korea Institute of Radiological and Medical Sciences (KIRAMS), funded by the Ministry of Science and ICT, Republic of Korea (No. 50547-2020) and the National Research Foundation of Korea (NRF) Grant funded by the Korea government (Ministry of Science and ICT) (No. 2019M2D2A1A02057204, 2020R1A2C2102492).
Author information
Authors and Affiliations
Contributions
This study was designed by C-HL, IL, S-KW, WK, KIK, KCL, KS, and SML. Data acquisition and analysis was performed by C-HL, WK, and S-KW. C-HL and IL wrote the manuscript draft. All authors reviewed and edited this final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, CH., Lim, I., Woo, SK. et al. Targeted alpha immunotherapy of CD20-positive B-cell lymphoma model: dosimetry estimate of 225Ac-DOTA-rituximab using 64Cu-DOTA-rituximab. Ann Nucl Med 35, 639–647 (2021). https://doi.org/10.1007/s12149-021-01607-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-021-01607-6