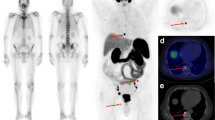
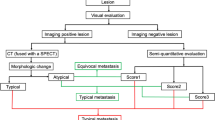
Abstract
Objectives
Gallium-68-labeled prostate-specific membrane antigen (Ga-PSMA) positron emission tomography (PET)/computed tomography (CT) is a valuable diagnostic tool for the detection of bone metastases in patients with prostate cancer (PCa). However, bone scintigraphy (BS) with technetium-labeled diphosphonates is cheap and widely available for the same patient population. PSMA PET comes with a cost, and financial constraints in the present economic environment may require its more selective use. In this study, we aimed to compare the diagnostic performance of BS with Ga-PSMA PET/CT for the detection of bone metastases in patients with PCa and correlate the results with various clinical and biochemical variables.
Materials and methods
Ninety-five patients who underwent Ga-PSMA PET/CT and BS within 3 months for newly diagnosed or recurrent PCa were extracted from our database. Lesion, region and patient-based analyses were performed. Clinical and imaging follow-up was used as the reference test. Results were compared with tumor grade, serum prostate-specific antigen (PSA), and alkaline phosphatase (ALP) values.
Results
On the patient-based analysis, 75% (42/56) and 98.2% (55/56) of the patients with bone metastases were correctly diagnosed by BS and Ga-PSMA PET, respectively. In 26/95 patients with equivocal lesions on BS, Ga-PSMA PET correctly reclassified skeletal involvement in 11 and excluded metastases in 15 patients BS missed bone metastases in 3 patients. The true-positive rate of BS in patients with serum ALP ≥ 120U/L and PSA ≥ 50 ng/ml was 95.8% and 87.5 respectively.
Conclusion
Ga-PSMA is superior to BS for the evaluation of metastatic disease in patients with PCa. However, BS can also detect bone metastases in patients with PCa with a minimum sensitivity of 75%. Biochemical data are helpful to select patients with a high pretest probability who should undergo BS first as a part of the initial workup from an economic point of view. Due to its higher cost, Ga-PSMA PET should be performed in a selective group of patients when BS results are inconclusive or metastasis-directed therapy is planned.




Similar content being viewed by others
References
Macedo F, Ladeira K, Pinho F, Saraiva N, Bonito N, Pinto L, et al. Bone metastases: an overview. Oncol Rev. 2017;11:321.
Mazzone E, Preisser F, Nazzani S, Tian Z, Bandini M, Gandaglia G, et al. Location of metastases in contemporary prostate cancer patients affects cancer-specific mortality. Clin Genitourin Cancer. 2018;16(5):376–84.
Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG Guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–29.
Rybak LD, Rosenthal DI. Radiological imaging for the diagnosis of bone metastases. Q J Nucl Med. 2001;45:53–64.
Maurer T, Eiber M, Schwaiger M, Gschwend JE, et al. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13:226–35.
Pyka T, Okamoto S, Dahlbender M, Tauber R, Retz M, Heck M, et al. Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:2114–211.
Schafer M, Bauder-Wust U, Leotta K, Zoller F, Mier W, Haberkorn U, et al. A dimerized urea-based inhibitor of the prostate-specific membrane antigen for 68Ga-PET imaging of prostate cancer. EJNMMI Res. 2012;2:23.
Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart HG, Hadaschik BA, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40:486–95.
Soloway MS, Hardeman SW, Hickey D, Raymond J, Todd B, Soloway S, Moinuddin M. Stratification of patients with metastatic prostate cancer based on extent of disease on initial scan. Cancer. 1988;61:195–202.
Eiber M, Herrmann K, Calais J, Hadaschik B, Giesel FL, Hartenbach M, et al. Prostate cancer molecular imaging standardized evaluation (PROMISE): proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med. 2018;59:469–78.
Araz M, Aras G, Kucuk ON. The role of 18F-NaF PET/CT in metastatic bone disease. J Bone Oncol. 2015;4:92–7.
Iagaru A, Mittra E, Dick DW, Sanjiv Sam Gambhir SS, et al. Prospective evaluation of 99mTc MDP scintigraphy, 18F NaF PET/CT, and 18F FDG PET/CT for detection of skeletal metastases. Mol Imaging Biol. 2012;14:252–9.
Bastawrous S, Bhargava P, Behnia F, Djang DSW, Haseley DR. Newer PET application with an old tracer: role of 18F-NaF skeletal PET/CT in oncologic practice. Radiographics. 2014;34:1295–316.
Lavalaye J, Kaldeway P, van Melick HH. Diffuse bone metastases on 68Ga-PSMA PET-CT in a patient with prostate cancer and normal bone scan. Eur J Nucl Med Mol Imaging. 2016;43:1563–4.
Rowe SP, Macura KJ, Ciarallo A, Mena E, Blackford A, Nadal S, et al. Comparison of prostate-specific membrane antigen-based 18F-DCFBC PET/CT to conventional imaging modalities for detection of hormone-naive and castration-resistant metastatic prostate cancer. J Nucl Med. 2016;57:46–53.
Tuncel M, Souvatzoglou M, Herrmann K, Stollfuss J, Schuster T, Weirich G, et al. [11C]Choline positron emission tomography/computed tomography for staging and restaging of patients with advanced prostate cancer. Nucl Med Biol. 2008;35:689–95.
Janssen JC, Woythal N, Meißner S, Prasad V, Brenner W, Diederichs G, et al. [68Ga]PSMA-HBED-CC uptake in osteolytic, osteoblastic, and bone marrow metastases of prostate cancer patients. Mol Imaging Biol. 2017;19(6):933–43.
Grubmüller B, Rasul S, Baltzer P, Fajkovic H, D'Andrea D, Berndl F, et al. Response assessment using [68Ga]Ga-PSMA ligand PET in patients undergoing systemic therapy for metastatic castration-resistant prostate cancer. Prostate. 2020;80:74–82.
Clement JM, Sweeney CJ. Evolving treatment of oligometastatic hormone-sensitive prostate cancer. J Oncol Pract. 2017;13:9–18.
Gartrell BA, Coleman R, Efstathiou E, Fizazi K, Logothetis CJ, Smith MR, et al. Metastatic prostate cancer and the bone: significance and therapeutic options. Eur Urol. 2015;68:850–8.
Decaestecker K, De Meerleer G, Lambert B, Delrue L, Fonteyne V, Claeys T, et al. Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat Oncol. 2014;9:135.
Berkovic P, De Meerleer G, Delrue L, Lambert B, Fonteyne V, Lumen N, et al. Salvage stereotactic body radiotherapy for patients with limited prostate cancer metastases: deferring androgen deprivation therapy. Clin Genitourin Cancer. 2013;11:27–322.
Muacevic A, Kufeld M, Rist C, Wowra B, Stief C, Staehler M, et al. Safety and feasibility of image-guided robotic radiosurgery for patients with limited bone metastases of prostate cancer. Urol Oncol. 2013;31:455–60.
Noguchi M, Kikuchi H, Ishibashi M, Noda S. Percentage of the positive area of bone metastasis is an independent predictor of disease death in advanced prostate cancer. Br J Cancer. 2003;88:195–201.
Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, Adnan A, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353–66.
Langley RR, Fidler IJ. The seed and soil hypothesis revisited—the role of tumor–stroma interactions in metastasis to different organs. Int J Cancer. 2011;128:2527–35.
Resnick MI. Hemodynamics of prostate bone metastases. In: Karr JP, Yamanaka H, editors. Prostate cancer and bone metastasis. Boston: Springer US; 1992. p. 77–81.
Dyrberg E, Hendel HW, Huynh THV, Klausen TW, Logager VB, Madsen C, et al. Ga-68-PSMA-PET/CT in comparison with F-18-fluoride PET/CT and whole body MRI for the detection of bone metastases in patients with prostate cancer: a prospective diagnostic accuracy study. Eur Radiol. 2018;29(3):1221–300.
Venkitaraman R, Sohaib A, Cook G. MRI or bone scan or both for staging of prostate cancer? J Clin Oncol. 2007;25:5837–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Caglar, M., Tuncel, M., Yildiz, E. et al. Bone scintigraphy as a gatekeeper for the detection of bone metastases in patients with prostate cancer: comparison with Ga-68 PSMA PET/CT. Ann Nucl Med 34, 932–941 (2020). https://doi.org/10.1007/s12149-020-01529-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-020-01529-9