Abstract
Objective
This study aimed to evaluate the accuracy of six threshold-based segmentation methods with different target-to-background ratios (TBR), images with different voxel sizes and image noise, in measuring metabolic volume (MV) and total glycolysis (TG).
Methods
A standard body phantom consisting of six spheres (inner diameters of 37, 28, 22, 17, 13, and 10 mm) was filled with 18F-FDG solution. The background radioactivity level was 2.65 kBq/mL, and the TBRs were 4 and 8. PET data were acquired for 30 min with list mode. PET data for 30 and 3 min were reconstructed with a three-dimensional ordered subset expectation maximization algorithm plus time-of-flight information with images with 2 and 4 mm isotropic voxels. The six methods examined were absolute standardized uptake value (SUV) of 2.5 (SUV2.5), 41%, 50%, adaptive 41%, and adaptive 50% thresholds of maximum SUV (Th41, Th50, ThA41, and ThA50, respectively); and the contrast-oriented algorithm (ThCOA). Segmented MV and TG were compared with the actual inner volume and expressed as percentages (%MVseg and %TGseg, respectively). In addition, the segmented MV was converted to the diameter, and the differences of it from the reference diameter were compared among six methods.
Results
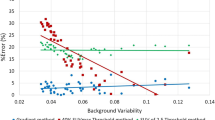
The ThCOA method yielded the most accurate measurements of %MVseg and %TGseg; the difference between %MVseg or %TGseg and its reference were smaller than 10% in 30-min and 15% in 3-min images, but the segmented contour was almost the same as the reference diameter. Measurements with Th50 and ThCOA were highly accurate for both %MVseg and %TGseg in the large spheres, and the adaptive threshold methods, including ThA41, ThA50, and ThCOA, were also highly accurate in the small spheres. The voxel sizes affected the accuracy of %MVseg and %TGseg with a TBR of 4 in any threshold-based methods.
Conclusions
Of the six threshold-based segmentation methods studied, ThCOA was the most accurate method for evaluating MV and TG and had only minor dependence on TBRs and sphere size. The small voxel sizes improved the variation of the accuracy in low TBR.






Similar content being viewed by others
References
Schöder H, Erdi YE, Larson SM, Yeung HWD. PET/CT: a new imaging technology in nuclear medicine. Eur J Nucl Med Mol Imaging. 2003;30:1419–37.
Townsend DW. Dual-modality imaging: combining anatomy and function. J Nucl Med. 2008;49:938–55.
Liu Y. FDG PET/CT for metastatic squamous cell carcinoma of unknown primary of the head and neck. Oral Oncol. 2019;92:46–51.
Groheux D, Cochet A, Humbert O, Alberini J-L, Hindie E, Mankoff D. 18F-FDG PET/CT for staging and restaging of breast cancer. J Nucl Med. 2016;57:17S–26S.
Duch J, Fuster D, Muñoz M, Fernández PL, Paredes P, Fontanillas M, et al. 18F-FDG PET/CT for early prediction of response to neoadjuvant chemotherapy in breast cancer. Eur J Nucl Med Mol Imaging. 2009;36:1551–7.
Lim R, Eaton A, Lee NY, Setton J, Ohri N, Rao S, et al. 18F-FDG PET/CT metabolic tumor volume and total lesion glycolysis predict outcome in oropharyngeal squamous cell carcinoma. J Nucl Med. 2012;53:1506–13.
Lee JW, Kang CM, Choi HJ, Lee WJ, Song SY, Lee J-H, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative 18F-FDG PET/CT in patients with pancreatic cancer. J Nucl Med. 2014;55:898–904.
Chung HW, Lee KY, Kim HJ, Kim WS, So Y. FDG PET/CT metabolic tumor volume and total lesion glycolysis predict prognosis in patients with advanced lung adenocarcinoma. J Cancer Res Clin Oncol. 2014;140:89–988.
Im HJ, Pak K, Cheon GJ, Kang KW, Kim SJ, Kim IJ, et al. Prognostic value of volumetric parameters of 18F-FDG PET in non-small-cell lung cancer: a meta-analysis. Eur J Nucl Med Mol Imaging. 2015;42:241–51.
Troost EGC, Schinagl DAX, Bussink J, Oyen WJG, Kaanders JHAM. Clinical evidence on PET-CT for radiation therapy planning in head and neck tumours. Radiother Oncol. 2010;96:328–34.
Ben-Haim S, Ell P. 18F-FDG PET and PET/CT in the evaluation of cancer treatment response. J Nucl Med. 2009;50:88–99.
Boellaard R, Krak NC, Hoekstra OS, Lammertsma AA. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J Nucl Med. 2004;45:1519–27.
Cheebsumon P, van Velden FHP, Yaqub M, Frings V, de Langen AJ, Hoekstra OS, et al. Effects of image characteristics on performance of tumor delineation methods: a test-retest assessment. J Nucl Med. 2011;52:1550–8.
Firouzian A, Kelly MD, Declerck JM. Insight on automated lesion delineation methods for PET data. EJNMMI Res. 2014;4:1–12.
Schaefer A, Kremp S, Hellwig D, Rübe C, Kirsch CM, Nestle U. A contrast-oriented algorithm for FDG-PET-based delineation of tumour volumes for the radiotherapy of lung cancer: Derivation from phantom measurements and validation in patient data. Eur J Nucl Med Mol Imaging. 2008;35:1989–99.
Cheebsumon P, Yaqub M, Van Velden FHP, Hoekstra OS, Lammertsma AA, Boellaard R. Impact of 18F-FDG PET imaging parameters on automatic tumour delineation: need for improved tumour delineation methodology. Eur J Nucl Med Mol Imaging. 2011;38:2136–44.
Ketabi A, Ghafarian P, Mosleh-Shirazi MA, Mahdavi SR, Rahmim A, Ay MR. Impact of image reconstruction methods on quantitative accuracy and variability of FDG-PET volumetric and textural measures in solid tumors. Eur Radiol. 2019;29:2146–56.
Hatt M, Cheze Le Rest C, Albarghach N, Pradier O, Visvikis D. PET functional volume delineation: a robustness and repeatability study. Eur J Nucl Med Mol Imaging. 2011;38:663–72.
Parkinson C, Evans M, Guerrero-Urbano T, Michaelidou A, Pike L, Barrington S, et al. Machine-learned target volume delineation of 18F-FDG PET images after one cycle of induction chemotherapy. Phys Med. 2019;61:85–93.
Conti M. Focus on time-of-flight PET: the benefits of improved time resolution. Eur J Nucl Med Mol Imaging. 2011;38:1147–57.
Boellaard R, Delgado-Bolton R, Oyen WJG, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54.
Koopman D, van Dalen JA, Lagerweij MCM, Arkies H, de Boer J, Oostdijk AHJ, et al. Improving the detection of small lesions using a state-of-the-art time-of-flight PET/CT system and small-voxel reconstructions. J Nucl Med Technol. 2015;43:21–7.
Li C-Y, Klohr S, Sadick H, Weiss C, Hoermann K, Schoenberg SO, et al. Effect of time-of-flight technique on the diagnostic performance of 18F-FDG PET/CT for assessment of lymph node metastases in head and neck squamous cell carcinoma. J Nucl Med Technol. 2014;42:181–7.
Morey AM, Noo F, Kadrmas DJ. Effect of using 2 mm voxels on observer performance for pet lesion detection. IEEE Trans Nucl Sci. 2016;63:1359–66.
Schaefer A, Kim YJ, Kremp S, Mai S, Fleckenstein J, Bohnenberger H, et al. PET-based delineation of tumour volumes in lung cancer: comparison with pathological findings. Eur J Nucl Med Mol Imaging. 2013;40:1233–44.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest were disclosed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nakaichi, T., Yamashita, S., Kawakami, W. et al. Accuracy of metabolic volume and total glycolysis among six threshold-based target segmentation algorithms. Ann Nucl Med 34, 583–594 (2020). https://doi.org/10.1007/s12149-020-01484-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-020-01484-5