Abstract
Objective
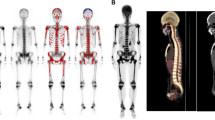
Artificial neural network (ANN) technology has been developed for clinical use to analyze bone scintigraphy with metastatic bone tumors. It has been reported to improve diagnostic accuracy and reproducibility especially in cases of prostate cancer. The aim of this study was to evaluate the diagnostic usefulness of quantitative bone scintigraphy with ANN in patients having breast cancer.
Patients and methods
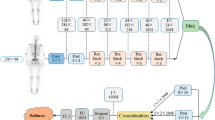
We retrospectively evaluated 88 patients having breast cancer who underwent both bone scintigraphy and 18F-fluorodeoxyglucose (FDG) positron-emission computed tomography/X-ray computed tomography (PET/CT) within an interval of 8 weeks between both examinations for comparison. The whole-body bone images were analyzed with fully automated software that was customized according to a Japanese multicenter database. The region of interest for FDG-PET was set to bone lesions in patients with bone metastasis, while the bone marrow of the ilium and the vertebra was used in patients without bone metastasis.
Results
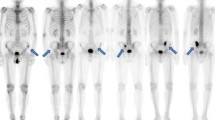
Thirty of 88 patients had bone metastasis. Extent of disease, bone scan index (BSI) which indicate severity of bone metastasis, the maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), total lesion glycolysis (TLG), and serum tumor markers in patients with bone metastasis were significantly higher than those in patients without metastasis. The Kaplan–Meier survival curve showed that the overall survival of the lower BSI group was longer than that with the higher BSI group in patients with visceral metastasis. In the multivariate Cox proportional hazard model, BSI (hazard ratio (HR): 19.15, p = 0.0077) and SUVmax (HR: 10.12, p = 0.0068) were prognostic factors in patients without visceral metastasis, while the BSI was only a prognostic factor in patients with visceral metastasis (HR: 7.88, p = 0.0084), when dividing the sample into two groups with each mean value in patients with bone metastasis.
Conclusion
BSI, an easily and automatically calculated parameter, was a well prognostic factor in patients with visceral metastasis as well as without visceral metastasis from breast cancer.




Similar content being viewed by others
References
Rubens RD. Bone metastases–the clinical problem. Eur J Cancer. 1998;34(2):210–3.
Zeintl J, et al. Quantitative accuracy of clinical 99mTc SPECT/CT using ordered-subset expectation maximization with 3-dimensional resolution recovery, attenuation, and scatter correction. J Nucl Med. 2010;51(6):921–8.
Fukuoka M, et al. Comparison of diagnostic value of I-123 MIBG and high-dose I-131 MIBG scintigraphy including incremental value of SPECT/CT over planar image in patients with malignant pheochromocytoma/paraganglioma and neuroblastoma. Clin Nucl Med. 2011;36(1):1–7.
Erdi YE, et al. Quantitative bone metastases analysis based on image segmentation. J Nucl Med. 1997;38(9):1401–6.
Imbriaco M, et al. A new parameter for measuring metastatic bone involvement by prostate cancer: the Bone Scan Index. Clin Cancer Res. 1998;4(7):1765–72.
Zafeirakis AG, Papatheodorou GA, Limouris GS. Clinical and imaging correlations of bone turnover markers in prostate cancer patients with bone only metastases. Nucl Med Commun. 2010;31(3):249–53.
Wakabayashi H, et al. Bone scintigraphy as a new imaging biomarker: the relationship between bone scan index and bone metabolic markers in prostate cancer patients with bone metastases. Ann Nucl Med. 2013;27(9):802–7.
Nakajima K, et al. Enhanced diagnostic accuracy for quantitative bone scan using an artificial neural network system: a Japanese multi-center database project. EJNMMI Res. 2013;3(1):83.
Iwase T, et al. The relationship between skeletal-related events and bone scan index for the treatment of bone metastasis with breast cancer patients. Medicine (Baltimore). 2014;93(28):e269.
Soloway MS, et al. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer. 1988;61(1):195–202.
Ethical Guidelines for Medical and Health Research Involving Human Subjects. Public notice of the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare No. 3 of 2014.
Ulmert D, et al. A novel automated platform for quantifying the extent of skeletal tumour involvement in prostate cancer patients using the Bone Scan Index. Eur Urol. 2012;62(1):78–84.
Sjostrand K, Ohlsson M, Edenbrandt L. Statistical regularization of deformation fields for atlas-based segmentation of bone scintigraphy images. Med Image Comput Comput Assist Interv. 2009;12(Pt 1):664–71.
Boellaard R, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–54.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45.
Sadik M, et al. Improved classifications of planar whole-body bone scans using a computer-assisted diagnosis system: a multicenter, multiple-reader, multiple-case study. J Nucl Med. 2009;50(3):368–75.
Tokuda O, et al. Investigation of computer-aided diagnosis system for bone scans: a retrospective analysis in 406 patients. Ann Nucl Med. 2014;28(4):329–39.
Coleman RE. Bisphosphonates: clinical experience. Oncologist. 2004;9(Suppl 4):14–27.
Norgaard M, et al. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999–2007). J Urol. 2010;184(1):162–7.
Jensen AO, et al. Incidence of bone metastases and skeletal-related events in breast cancer patients: a population-based cohort study in Denmark. BMC Cancer. 2011;11:29.
Khatcheressian JL, et al. Breast cancer follow-up and management after primary treatment: American Society of clinical oncology clinical practice guideline update. J Clin Oncol. 2013;31(7):961–5.
Sadik M, et al. Quality of planar whole-body bone scan interpretations–a nationwide survey. Eur J Nucl Med Mol Imaging. 2008;35(8):1464–72.
Krammer J, et al. (18)F-FDG PET/CT for initial staging in breast cancer patients–Is there a relevant impact on treatment planning compared to conventional staging modalities? Eur Radiol. 2015;25(8):2460–9.
Groheux D, et al. F-FDG PET/CT in the early prediction of pathological response in aggressive subtypes of breast cancer: review of the literature and recommendations for use in clinical trials. Eur J Nucl Med Mol Imaging. 2016;43(5):983–93.
Ishiba T, et al. Efficiency of fluorodeoxyglucose positron emission tomography/computed tomography to predict prognosis in breast cancer patients received neoadjuvant chemotherapy. Springerplus. 2015;4:817.
Murakami R, et al. FDG-PET/CT in the diagnosis of recurrent breast cancer. Acta Radiol. 2012;53(1):12–6.
Son SH, et al. Whole-body metabolic tumor volume, as determined by (18)F-FDG PET/CT, as a prognostic factor of outcome for patients with breast cancer who have distant metastasis. AJR Am J Roentgenol. 2015;205(4):878–85.
Rong J, et al. Comparison of 18 FDG PET-CT and bone scintigraphy for detection of bone metastases in breast cancer patients. A meta-analysis. Surg Oncol. 2013;22(2):86–91.
Shintawati R, et al. Evaluation of bone scan index change over time on automated calculation in bone scintigraphy. Ann Nucl Med. 2015;29(10):911–20.
Acknowledgements
The authors thank Dr. Masafumi Inokuchi for his precise description of patients’ clinical history
Funding
None
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
K. Nakajima has a collaborative research work with FUJIFILM Toyama Chemical for developing BONENAVI software. Other authors have declared no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Inaki, A., Nakajima, K., Wakabayashi, H. et al. Fully automated analysis for bone scintigraphy with artificial neural network: usefulness of bone scan index (BSI) in breast cancer. Ann Nucl Med 33, 755–765 (2019). https://doi.org/10.1007/s12149-019-01386-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-019-01386-1