Abstract
Objective
Complete surgical resection of metastatic sites has been shown to prolong survival in select patients with oligometastatic RCC. This treatment strategy is dependent upon the accurate characterization of a patient’s extent of disease. The objective of this study was to explore the utility of PSMA-targeted 18F-DCFPyL PET/CT in patients with presumed oligometastatic clear cell RCC.
Methods
This is a subset analysis of a prospective study in which patients with RCC were imaged with 18F-DCFPyL PET/CT (ClinicalTrials.gov identifier NCT02687139). In the present analysis, patients with oligometastatic clear cell RCC, defined as ≤ 3 metastatic lesions on conventional imaging, were evaluated. 18F-DCFPyL PET/CT scans were reviewed for sites of disease and compared to conventional imaging.
Results
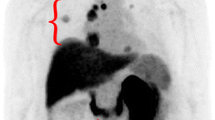
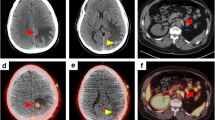
The final cohort included 14 patients with oligometastatic clear cell RCC. Conventional imaging revealed 21 metastatic lesions and 3 primary tumors. 18F-DCFPyL PET/CT detected 29 sites of metastatic disease and 3 primary tumors. Of the 21 metastatic lesions detected on conventional imaging, 17 (81.0%) had radiotracer uptake. Additionally, all 3 primary tumors had radiotracer uptake. In 4 (28.6%) patients a total of 12 more lesions were identified on 18F-DCFPyL PET/CT than conventional imaging. Notably, 3 (21.4%) patients were no longer considered oligometastatic. The detection rates of conventional imaging and 18F-DCFPyL PET/CT for identifying sites of disease were 66.7% and 88.9%, respectively.
Conclusions
PSMA-targeted PET/CT appears to aid in the identification of patients with oligometastatic clear cell RCC. If borne out in future studies, this suggests that PSMA-targeted imaging has the potential to help select candidates for metastasis-directed therapy.


Similar content being viewed by others
References
Psutka SP, Master VA. Role of metastasis-directed treatment in kidney cancer. Cancer. 2018;124(18):3641–55.
Dabestani S, Marconi L, Hofmann F, et al. Local treatments for metastases of renal cell carcinoma: a systematic review. Lancet Oncol. 2014;15(12):e549–561.
Zaid HB, Parker WP, Safdar NS, et al. Outcomes following complete surgical metastasectomy for patients with metastatic renal cell carcinoma: a systematic review and meta-analysis. J Urol. 2017;197(1):44–9.
Brufau BP, Cerqueda CS, Villalba LB, Izquierdo RS, Gonzalez BM, Molina CN. Metastatic renal cell carcinoma: radiologic findings and assessment of response to targeted antiangiogenic therapy by using multidetector CT. Radiographics. 2013;33(6):1691–716.
Chang SS, Reuter VE, Heston WDW, Bander NH, Grauer LS, Gaudin PB. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59(13):3192–8.
Chang SS, O'Keefe DS, Bacich DJ, Reuter VE, Heston WDW, Gaudin PB. Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin Cancer Res. 1999;5(10):2674–81.
Chang SS, Gaudin PB, Reuter VE, O'Keefe DS, Bacich DJ, Heston WDW. Prostate-specific membrane antigen: much more than a prostate cancer marker. Mol Urol. 1999;3(3):313–9.
Chang SS, Reuter VE, Heston WD, Gaudin PB. Metastatic renal cell carcinoma neovasculature expresses prostate-specific membrane antigen. Urology. 2001;57(4):801–5.
Rowe SP, Gorin MA, Hammers HJ, Pomper MG, Allaf ME, Javadi MS. Detection of 18F-FDG PET/ct occult lesions with 18F-DCFPyL PET/CT in a patient with metastatic renal cell carcinoma. Clin Nucl Med. 2016;41(1):83–5.
Rowe SP, Gorin MA, Hammers HJ, et al. Imaging of metastatic clear cell renal cell carcinoma with PSMA-targeted 18F-DCFPyL PET/CT. Ann Nucl Med. 2015;29(10):877–82.
Sasikumar A, Joy A, Nanabala R, Unni M, Tk P. Complimentary pattern of uptake in 18F-FDG PET/CT and 68Ga-prostate-specific membrane antigen PET/CT in a case of metastatic clear cell renal carcinoma. Clin Nucl Med. 2016;41(12):e517–e519519.
Gorin MA, Rowe SP, Hooper JE, et al. PSMA-targeted 18F-DCFPyL PET/CT imaging of clear cell renal cell carcinoma: results from a rapid autopsy. Eur Urol. 2017;71(1):145–6.
Rhee H, Blazak J, Tham CM, et al. Pilot study: use of gallium-68 PSMA PET for detection of metastatic lesions in patients with renal tumour. EJNMMI Res. 2016;6(1):76.
Yin Y, Campbell SP, Markowski MC, Pierorazio PM, Pomper MG, Allaf ME, Rowe SP, Gorin MA. Inconsistent detection of sites of metastatic non-clear cell renal cell carcinoma with PSMA-targeted [18F]DCFPyL PET/CT. Mol Imaging Biol. 2019;21(3):567–73.
Ravert HT, Holt DP, Chen Y, et al. An improved synthesis of the radiolabeled prostate-specific membrane antigen inhibitor, [18F]DCFPyL. J Label Comp Radiopharm. 2016;59(11):439–50.
Sheikhbahaei S, Afshar-Oromieh A, Eiber M, et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur J Nucl Med Mol Imaging. 2017;44(12):2117–366.
Heng DYC, Xie WL, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a Largemulticenter study. J Clin Oncol. 2009;27(34):5794–9.
Network NCC. National Comprehensive Cancer Network. Kidney. Version 2.2019. 2019; https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf. Accessed 28 Jan 2019.
Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67(5):913–24.
Campbell SP, Baras AS, Ball MW, et al. Low levels of PSMA expression limit the utility of 18F-DCFPyL PET/CT for imaging urothelial carcinoma. Ann Nucl Med. 2018;32(1):69–74.
Stillebroer AB, Mulders PF, Boerman OC, Oyen WJ, Oosterwijk E. Carbonic anhydrase IX in renal cell carcinoma: implications for prognosis, diagnosis, and therapy. Eur Urol. 2010;58(1):75–83.
Divgi CR, Uzzo RG, Gatsonis C, et al. Positron emission tomography/computed tomography identification of clear cell renal cell carcinoma: results from the REDECT trial. J Clin Oncol. 2013;31(2):187–94.
Hekman MCH, Rijpkema M, Aarntzen EH, et al. Positron emission tomography/computed tomography with 89Zr-girentuximab can aid in diagnostic dilemmas of clear cell renal cell carcinoma suspicion. Eur Urol. 2018;74(3):257–60.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MGP is a co-inventor on a US Patent covering 18F-DCFPyL and as such is entitled to a portion of any licensing fees and royalties generated by this technology. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict-of-interest policies. MAG has served as a consultant to Progenics Pharmaceuticals, the licensee of 18F-DCFPyL. SPR, MAG, and MGP have received research support from Progenics Pharmaceuticals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meyer, A.R., Carducci, M.A., Denmeade, S.R. et al. Improved identification of patients with oligometastatic clear cell renal cell carcinoma with PSMA-targeted 18F-DCFPyL PET/CT. Ann Nucl Med 33, 617–623 (2019). https://doi.org/10.1007/s12149-019-01371-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-019-01371-8