Abstract
Objective
The purpose of this study was to evaluate the performance characteristics and safety of florbetapir (I8F) positron emission tomography (PET) in patients with Alzheimer’s disease (AD) and mild cognitive impairment (MCI) and cognitively normal (CN) control patients from Japan.
Methods
Florbetapir (I8F) PET was obtained in 48 subjects (15 AD patients, 15 MCI patients, and 18 CNs) within a multicenter phase 2/3 study. Amyloid burden was assessed visually and classified as positive or negative for pathologic levels of amyloid aggregation, blind to diagnostic classification. Cerebral to cerebellar standardized uptake value ratios (SUVRs) were determined from the florbetapir (I8F) PET images. Safety was assessed by monitoring adverse events, vital signs, clinical laboratory assessments, and electrocardiograms. Demographic variables and cognitive scales were summarized by using descriptive statistics for each group. Fisher’s exact test and one-way analysis of variance were used to compare amyloid positivity and mean SUVRs, respectively, between diagnostic groups.
Results
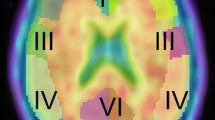
Florbetapir (I8F) PET was rated visually amyloid positive in 80.0 % of AD patients, 33.3 % of MCI patients, and 16.7 % of CNs. Mean SUVRs were highest in the AD group and lowest in the CN group for each brain region (P < 0.01) and globally (P < 0.05). Kappa statistics showed strong inter-reader agreement (Fleiss’ kappa = 0.82) and individual reader’s agreement with the majority of readers (kappa ranged from 0.79 to 1.0). Seventeen of the 48 subjects (35.4 %) were Apolipoprotein E genotype ε4 positive, which included 10 subjects in the AD group and 7 subjects in the MCI group. A total of 6 subjects (5 of whom were in the CN group) had at least 1 treatment-emergent adverse event (TEAE).
Conclusions
These data indicate that amyloid positivity increased with diagnostic category (CN < MCI < AD) and are consistent with expected rates of amyloid positivity among individuals with clinical diagnoses of AD and MCI. In addition, these results were similar to those obtained in United States studies. Florbetapir (18F) was safe and well tolerated. The reliability of both qualitative and quantitative assessments of florbetapir (18F) in this study population provides support for potential use in clinical settings in Japan.


Similar content being viewed by others
References
Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–7.
Anderson GF, Hussey PS. Population aging: a comparison among industrialized countries. Health Aff (Millwood). 2000;19(3):191–203.
Asada T. Prevalence of dementia in Japan: past, present and future. Rinsho Shinkeigaku. 2012;52(11):962–4.
Health and Labour Sciences Research Grant. Dementia research project. 2013. http://www.tsukuba-psychiatry.com/wp-content/uploads/2013/06/H24Report_Part1.pdf. Accessed 16 Jan 2014.
Knopman DS, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1143–53.
Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer’s disease. Neurobiol Aging. 1997;18(4 Suppl):S1–S2.
Rowe CC, Villemagne VL. Amyloid imaging with PET in early Alzheimer disease diagnosis. Med Clin N Am. 2013;97(3):377–98.
Vandenberghe R, Adamczuk K, Dupont P, Laere KV, Chetelat G. Amyloid PET in clinical practice: its place in the multidimensional space of Alzheimer’s disease. Neuroimage Clin. 2013;2:497–511.
Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann Neurol. 2004;55(3):306–19.
Nordberg A. Amyloid imaging in early detection of Alzheimer’s disease. Neurodegener Dis. 2010;7(1–3):136–8.
Koole M, Lewis DM, Buckley C, Nelissen N, Vandenbulcke M, Brooks DJ, et al. Whole-body biodistribution and radiation dosimetry of 18F-GE067: a radioligand for in vivo brain amyloid imaging. J Nucl Med. 2009;50(5):818–22.
Rowe CC, Ackerman U, Browne W, Mulligan R, Pike KL, O’Keefe G, et al. Imaging of amyloid beta in Alzheimer’s disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol. 2008;7(2):129–35.
Small GW, Kepe V, Ercoli LM, Siddarth P, Bookheimer SY, Miller KJ, et al. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med. 2006;355(25):2652–63.
Choi SR, Golding G, Zhuang Z, Zhang W, Lim N, Hefti F, et al. Preclinical properties of 18F-AV-45: a PET agent for Abeta plaques in the brain. J Nucl Med. 2009;50(11):1887–94.
Wong DF, Rosenberg PB, Zhou Y, Kumar A, Raymont V, Ravert HT, et al. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir [corrected] F18). J Nucl Med. 2010;51(6):913–20.
Pontecorvo MJ, Mintun MA. PET amyloid imaging as a tool for early diagnosis and identifying patients at risk for progression to Alzheimer’s disease. Alzheimers Res Ther. 2011;3(2):11.
Tateno A, Sakayori T, Kawashima Y, Higuchi M, Suhara T, Mizumura S, et al. Comparison of imaging biomarkers for Alzheimer’s disease: amyloid imaging with [18 F]florbetapir positron emission tomography and magnetic resonance imaging voxel-based analysis for entorhinal cortex atrophy. Int J Geriatr Psychiatry. 2015;30(5):505–13.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–44.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98.
Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S33–9.
Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–4.
Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141(11):1356–64.
Wechsler D. Wechsler memory scale-revised. San Antonio: The Psychological Corporation; 1987.
Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–65.
Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 2012;11(8):669–78.
Amyvid prescribing information. 2012. http://pi.lilly.com/us/amyvid-uspi.pdf. Accessed 4 June 2014.
Joshi AD, Pontecorvo MJ, Clark CM, Carpenter AP, Jennings DL, Sadowsky CH, et al. Performance characteristics of amyloid PET with florbetapir F18 in patients with Alzheimer’s disease and cognitively normal subjects. J Nucl Med. 2012;53(3):378–84.
Fleisher AS, Chen K, Liu X, Roontiva A, Thiyyagura P, Ayutyanont N, et al. Using positron emission tomography and florbetapir F18 to image cortical amyloid in patients with mild cognitive impairment or dementia due to Alzheimer disease. Arch Neurol. 2011;68(11):1404–11.
Joshi AD, Pontecorvo MJ, Lu M, Grundman M, Skovronsky DM, Mintun MA, et al. A semi-automated method for quantification of florbetapir F18 PET images. J Nucl Med. 2015 (accepted).
Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA. 2011;305(3):275–83.
Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64(5):834–41.
Gearing M, Schneider JA, Rebeck GW, Hyman BT, Mirra SS. Alzheimer’s disease with and without coexisting Parkinson’s disease changes: apolipoprotein E genotype and neuropathologic correlates. Neurology. 1995;45(11):1985–90.
Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM. Neuropathological and neuropsychological changes in “normal” aging: evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol. 1998;57(12):1168–74.
Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62(11):1087–95.
Lim A, Tsuang D, Kukull W, Nochlin D, Leverenz J, McCormick W, et al. Clinico-neuropathological correlation of Alzheimer’s disease in a community-based case series. J Am Geriatr Soc. 1999;47(5):564–9.
Petersen RC, Parisi JE, Dickson DW, Johnson KA, Knopman DS, Boeve BF, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63(5):665–72.
Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45(3):358–68.
Rasmusson DX, Brandt J, Steele C, Hedreen JC, Troncoso JC, Folstein MF. Accuracy of clinical diagnosis of Alzheimer disease and clinical features of patients with non-Alzheimer disease neuropathology. Alzheimer Dis Assoc Disord. 1996;10(4):180–8.
Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. “Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000;55(3):370–6.
Johnson KA, Sperling RA, Gidicsin CM, Carmasin JS, Maye JE, Coleman RE, et al. Florbetapir (F18-AV-45) PET to assess amyloid burden in Alzheimer’s disease dementia, mild cognitive impairment, and normal aging. Alzheimers Dement. 2013;9(5 Suppl):S72–83.
Doraiswamy PM, Sperling RA, Coleman RE, Johnson KA, Reiman EM, Davis MD, et al. Amyloid-beta assessed by florbetapir F18 PET and 18-month cognitive decline: a multicenter study. Neurology. 2012;79(16):1636–44.
Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci USA. 2009;106(16):6820–5.
Lim A, Tsuang D, Kukull W, Nochlin D, Leverenz J, McCormick W, et al. Clinico-neuropathological correlation of Alzheimer’s disease in a community-based case series. J Am Geriatr Soc. 1999;47(5):564–9.
Mayeux R, Saunders AM, Shea S, Mirra S, Evans D, Roses AD, et al. Utility of the apolipoprotein E genotype in the diagnosis of Alzheimer’s disease. Alzheimer’s disease centers consortium on Apolipoprotein E and Alzheimer’s disease. N Engl J Med. 1998;338(8):506–11.
Ranginwala NA, Hynan LS, Weiner MF, White CL III. Clinical criteria for the diagnosis of Alzheimer disease: still good after all these years. Am J Geriatr Psychiatry. 2008;16(5):384–8.
Acknowledgments
We thank the Institute of Biomedical Research and Innovation, The University of Tokyo, and Nippon Medical School; each acted as a contract manufacturer of florbetapir (18F) as an investigational medical product.
We also thank the staff of the all the sites, in particular, Takuya Arai (The University of Tokyo Hospital), Masahiro Sasaki (Institute of Biomedical Research and Innovation), and Kazuyoshi Honjo (Nippon Medical School) for manufacturing and quality control.
Lastly, the authors would like to thank Shannon Gardell, PhD (inVentiv Health Clinical, LLC) for assistance with preparation of the manuscript and Angela Lorio, ELS (inVentiv Health Clinical, LLC) for editorial assistance.
CN was a full-time employee of, and minor stockholder in, Eli Lilly and Company at the time of this work and is now employed by Kyoto University Hospital. YT is a full-time employee of, and minor stockholder in, Eli Lilly and Company. AA, ADJ, ML, and MJP are employees of Avid Radiopharmaceuticals, a wholly owned subsidiary of Eli Lilly. CB was an employee of Avid Radiopharmaceuticals at the time of this work and is now employed by Spectrum Dynamics. AI has received research funding from Jannsen Pharmaceuticals. TM has served as a clinical trial representative for and received research funding from Eli Lilly and Company. MS has served as a clinical trial representative for Eli Lilly and Company/Avid Radiopharmaceuticals. YO has received research funding from Quintiles.
This research was totally funded by Eli Lilly and Company and/or any of its subsidiaries.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial Registration: NCT01662882.
Rights and permissions
About this article
Cite this article
Namiki, C., Takita, Y., Iwata, A. et al. Imaging characteristics and safety of florbetapir (18F) in Japanese healthy volunteers, patients with mild cognitive impairment and patients with Alzheimer’s disease. Ann Nucl Med 29, 570–581 (2015). https://doi.org/10.1007/s12149-015-0978-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-015-0978-2