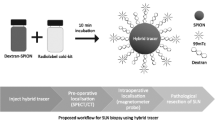
Abstract
Objective
The aim of this work was to radiolabel and bioevaluate the technetium-99m labeled dextran dicysteine mannose (DCCM) [99mTc(CO)3]-DCCM for sentinel lymph node detection.
Methods
Dextran dicysteine mannose was radiolabeled using the carbonyl method. Various parameters were studied such as in vitro stability at room temperature up to 5 h, protein binding and partition coefficient. Bioevaluation was performed in a rabbit model by developing images under a gamma camera at various time intervals. Biodistribution was performed in Wistar rat models (n = 3) by dissection and measurement of percent injected dose in various body organs, at 60 and 180 min post-injection intervals. Biodistribution was performed in two different groups of animals: in the first group, the radiolabeled compound was injected at a concentration of 200 μg/ml, thus delivering 10 μg radiolabeled compound at the site of injection; in the second group, the radiolabeled compound was injected at a concentration of 50 μg/ml, delivering 2.5 μg radiolabeled compound at the site of injection.
Results
Radiolabeling efficacy was 97.5 ± 1 % which remained quite stable till 5 h. Protein binding data show that 71.1 ± 5 % drug exhibited binding with blood proteins. Partition coefficient results show that our radiopharmaceutical is quite hydrophilic in nature. It can be inferred from the imaging data that sentinel node can be visualized within 30 min post-injection. Rat dissection data showed that when the radiolabeled compound was injected at a concentration of 50 μg/ml, at 60 min post-injection, ~2.85 % of activity was retained in the sentinel node with a significantly less accumulation, e.g., ~0.12 %, in the secondary node, which resulted in very high popliteal extraction (PE) value, e.g., ~98 %. At 180 min post-injection, 2.46 ± 0.29 % was found to be retained in the sentinel node and PE (99.64 ± 0.23 %), thus resulting in almost complete washout from the secondary node (0.05 ± 0.01 %).
Conclusion
The study demonstrates that radiolabeled DCCM might be a successful radiopharmaceutical for sentinel node detection.



Similar content being viewed by others
References
Mariani G, Moresco L, Viale G, Villa G, Bagnasco M, Canavese G, et al. Radioguided sentinel lymph node biopsy in breast cancer surgery. J Nucl Med. 2001;42:1198–215.
Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53:283–318.
Chakera AH, Hesse B, Burak Z, Ballinger JR, Britten A, Caraco C, et al. EANM-EORTC general recommendations for sentinel node diagnostics in melanoma. Eur J Nucl Med Mol Imaging. 2009;36:1713–42.
Wieler H, Bartenstein P, Becker HP, Bell E, Decker P, Jacob R, et al. Guideline for therapy of malign ant thyroid tumours. Nuklearmedizin. 2004;43:121–3.
Kogan MJ, Olmedo I, Hosta L, Guerrero AR, Cruz LJ, Albericio F. Peptides and metallic nanoparticles for biomedical applications. Nanomedicine (London). 2007;2(3):287–306.
Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–7.
Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug Chem. 2004;15:897–900.
Wallace AM, Hoh CK, Vera DR, Darrah DD, Schulteis G. Lymphoseek: a molecular radiopharmaceutical for sentinel node detection. Ann Surg Oncol. 2003;10(5):531–8.
Mindt TL, Struthers H, Brans L, Anguelov T, Schweinsberg C, Maes V, et al. “Click to chelate”: synthesis and installation of metal chelates into biomolecules in a single step. J Am Chem Soc. 2006;128:15096–7.
Alberto R, Ortner K, Wheatly N, Schibli R, Schubiger AP. Synthesis and properties of boranocarbonate: a convenient in situ co source for the aqueous preparation of [99mTc(OH2)3(CO)3]+. J Am Chem Soc. 2001;123:3135–6.
Alberto R, Schibli R, Egli A, Schubiger AP, Abram U, Kaden TA. Novel organometallic aqua complex of technetium for the labeling of biomolecules: synthesis of [99mTc(OH2)3(CO)3]+ from [99mTcO4]− in aqueous solution and its reaction with a bifunctional ligand. J Am Chem Soc. 1998;120:7987–8.
Vera DR, Wallace AM, Hoh CK, Mattrey RF. A synthetic macromolecule for sentinel node detection: [99mTc]DTPA-mannosyl-dextran. J Nucl Med. 2001;42:951–9.
Steer CJ, Ashwell G. Receptor-mediated endocytosis: mechanisms, biologic function, and molecular properties. 2nd ed. Philadelphia: WB Saunders; 1990.
Vera DR, Buonocore MH, Wisner ER, Katzberg RW, Stadalnik RC. A molecular receptor-binding contrast agent for magnetic resonance imaging of the liver. Acad Radiol. 1995;2:497–507.
Vera DR, Topcu SJ, Stadalnik RC. In vitro quantification of asialoglycoprotein receptor density from human hepatic microsamples. Methods Enzymol. 1995;247:394–402.
Vera DR, Wisner ER, Stadalnik RC. Sentinel node imaging via a nonparticulate receptor-binding radiotracer. J Nucl Med. 1997;38:530–5.
Thoren L. Dextran as a plasma volume substitute. In: Jamieson GA, Greenwalt TJ, editors. Blood substitutes and plasma expenders. New York: Alan R Liss; 1978. p. 265–82.
de Belder D. Medical application of dextran and its derivatives. In: Dumitriu S, editor. Polysaccharides in medicinal applications. New York: Marcel Dekker; 1996. p. 505–24.
Porter CJH. Drug delivery to the lymphatic system. Crit Rev Ther Drug Carrier Syst. 1997;14:333–93.
Haigh PI, Guiliano AE. Role of sentinel lymph node dissection in breast cancer. Ann Med. 2000;32:51–6.
Hirsch JI, Tisnado J, Cho SR, Beachley MC. Use of isosulfan blue for identification of lymphatic vessels: experimental and clinical evaluation. AJR. 1982;139:1061–4.
Bárczai-Martos M, Kõrösy F. Preparation of acetobrome-sugars. Nature. 1950;165:369.
Chipowsky S, Lee YC. Synthesis of 1-thioaldosides having an amino group at the aglycon terminal. Carbohydr Res. 1973;31:339–46.
Lee YC, Stowell CP, Krantz MJ. 2-Imino-2-methoxylethyl-1-thioglycosides: new reagents for attaching sugars to proteins. Biochemistry. 1976;15:3956–63.
Nunez EGF, Teodoro R, Wiecek DP, da Silva NG, Martinlli JR, de Oliveira Filho RS. Size and specificity of radiopharmaceuticals for sentinel lymph node detection. Acta Radiol. 2011;52:774–8.
Subramanian S, Pandey U, Papadopoulos M, Pirmettis I, Venkatesh M, Samuel G. Studies toward the biological efficacy of 99mTc-labeled dextran-cysteine-mannose ([99mTc(CO)3]DCM20) for sentinel lymph node detection. Cancer Biother Radiopharm. 2012;27(6):365–70.
Acknowledgments
The authors highly acknowledge the financial support of International Atomic Energy Agency (IAEA) for funding this project under a Coordinated Research Programme (PAK/CRP-14599). We thank Dr. J Kornyei and his collaborators at the Institute of Izotopes Co. Ltd. Budapest, Hungary, for synthesizing and providing us the DCCM derivative and Adriano Duatti at IAEA for his technical support. The authors acknowledge the services of Mr. Khalid Mahmood, Senior Technologist, INMOL, for assisting in the imaging and biodistribution studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, I.U., Shahid, A., Ahmad, F. et al. Studying the biological feasibility of [99mTc(CO)3]-dextran-cysteine-cysteine-mannose as a potential molecular radiopharmaceutical for sentinel node detection. Ann Nucl Med 28, 248–256 (2014). https://doi.org/10.1007/s12149-013-0802-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-013-0802-9