Abstract
Introduction
Lately, 6-[18F]fluoro-l-DOPA (FDOPA) has found increase in its clinical demand for whole-body positron emission tomography (PET) scans, and two key issues in fulfilling this demand are the difficulties in producing FDOPA under the recently imposed PET drug good manufacturing practice (GMP) regulations and in providing it in the quality meeting the terms of major compendia. This paper describes the approaches for the GMP production of FDOPA and for the product testing to meet the standard of United States Pharmacopeia (USP) “Fluorodopa F 18 Injection.”
Methods
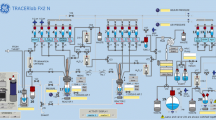
FDOPA was produced by the carrier-added electrophilic aromatic substitution reaction in the facility complying Pharmaceutical Inspection Cooperation Scheme clean room standard. The special aseptic handling technique was applied to minimize the bioburden. The product quality control followed all testing items and procedures, including three different settings of HPLC.
Results
The process yielded FDOPA average 2.60 ± 0.26 GBq (N = 22) in every batch. All qualities of the product were within the specifications described in the USP “Fluorodopa F 18 Injection.” The entire production was audited by the government authority and certified to comply with the latest PET drug GMP regulation.
Conclusion
Our efforts in producing FDOPA following all aspects of GMP requirements have resulted in a product with the USP quality and certified as GMP complied. The routine production yields enough doses for three to four whole-body scans in each batch. The issues discussed in the report provide good reference for producers planning in routine production for PET drugs that are not commonly produced or with complicated compendial quality control tests.


Similar content being viewed by others
References
Heiss WD, Hilker R. The sensitivity of 18-fluorodopa positron emission tomography and magnetic resonance imaging in Parkinson’s disease. Eur J Neurol. 2004;11:5–12.
Thobois S, Guillouet S, Broussolle E. Contributions of PET and SPECT to the understanding of the pathophysiology of Parkinson’s disease. Neurophysiol Clin. 2001;31:321–40.
Garnett ES, Firnau G, Nahmias C. Dopamine visualized in the basal ganglia of living man. Nature. 1983;305:137–8.
Minn H, Kauhanen S, Seppänen M, Nuutila P. 18F-FDOPA: a multiple-target molecule. J Nucl Med. 2009;50:1915–8.
Koopmans KP, Neels OC, Kema IP, Elsinga PH, Sluiter WJ, Vanghillewe K, et al. Improved staging of patients with carcinoid and islet cell tumors with 18F-dihydroxy-phenyl-alanine and 11C-5-hydroxy-tryptophan positron emission tomography. J Clin Oncol. 2008;26:1489–95.
Becherer A, Szabó M, Karanikas G, Wunderbaldinger P, Angelberger P, Raderer M, et al. Imaging of advanced neuroendocrine tumors with 18F-FDOPA PET. J Nucl Med. 2004;45:1161–7.
Lemaire C, Damhaut P, Plenevaux A, Comar D. Enantioselective synthesis of 6-[fluorine-18]-fluoro-l-dopa from no-carrier-added fluorine-18-fluoride. J Nucl Med. 1994;35:1996–2002.
Adam MJ, Ruth TJ, Grierson JR, Abeysekera B, Pate BD. Routine synthesis of l-[18F]6-fluorodopa with fluorine-18 acetyl hypofluorite. J Nucl Med. 1986;27:1462–6.
de Vries EFJ, Luurtsemaa G, Brüssermannb M, Elsinga PH, Vaalburg W. Fully automated synthesis module for the high yield one-pot preparation of 6-[18F]fuoro-l-DOPA. Appl Radiat Isot. 1999;51:389–94.
Chang CW, Wang HE, Lin HM, Chtsai CS, Chen JB, Liu RS. Robotic synthesis of 6-[18F]fluoro-l-dopa. Nucl Med Commun. 2000;21:799–802.
Food and Drug Administration Center for Drug Evaluation and Research. Guidance: PET Drugs—Current Good Manufacturing Practice (CGMP). In: Guidances (Drugs)/Current Good Manufacturing Practices (CGMPs). United States Department of Health and Human Services. 2005. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070306.pdf. Accessed 29 July 2010.
European Commission Enterprise and Industry. Volume 4: EU guidelines to good manufacturing practice: medicinal products for human and veterinary use; Annex 3: Manufacture of Radiopharmaceuticals. In: EudraLex, The Rules Governing Medicinal Products in the European Union. European Commission. 2008. http://ec.europa.eu/health/files/eudralex/vol-4/pdfs-en/anx03_en.pdf. Accessed 6 Aug 2010.
Pharmaceutical Inspection Cooperation Scheme. Annex 3: Manufacture of radiopharmaceuticals In: PE 009-9 (Annexes) Guide to good manufacturing practice for medicinal products, Annexes. Pharmaceutical Inspection Convention. 2009. http://www.picscheme.org/publication.php?id=4. Accessed 29 July 2010.
United States Pharmacopeial Convention. Monographs: Fluorodopa F 18 Injection. In: USP31/NF26, vol 2. 2008. p. 2193–4.
Pharmaceutical Inspection Cooperation Scheme. Annex 1: Manufacture of sterile medicinal products. In: PE 009-9 (Annexes) Guide to good manufacturing practice for medicinal products, Annexes. Pharmaceutical Inspection Convention. 2009. http://www.picscheme.org/publication.php?id=4. Accessed 29 July 2010.
United States Pharmacopeial Convention. Reagents, indicators, and solutions: chromatographic reagents. In: USP31/NF26, vol 1. 2008. p. 810–2.
Boué J, Audebert R, Quivoron C. Direct resolution of alpha-amino acid enantiomers by ligand exchange: stereoselection mechanism on silica packings coated with a chiral polymer. J Chromatogr. 1981;204:185–93.
Lemaire C, Guillaume M, Cantineau R, Christiaens L. No-carrier-added regioselective preparation of 6-[18F]fluoro-l-dopa. J Nucl Med. 1990;31:1247–51.
Ishiwata K, Ido T, Vaalburg W. Increased amount of d-enantiomer dependent on alkaline concentration in the synthesis of l-[methyl-11C]methionine. Appl Radiat Isot. 1988;39:311–4.
Schlauch M, Volk FJ, Fondekar KP, Wede J, Frahm AW. Enantiomeric and diastereomeric high-performance liquid chromatographic separation of cyclic beta-substituted alpha-amino acids on a copper(II)-d-penicillamine chiral stationary phase. J Chromatogr. 2000;897:145–52.
Pfeffer M, Gelbe B, Hampe P, Steinberg B, Walenciak-Reddel E, Woicke B, et al. Sensitive fluorescence labeling for analysis of organotin compounds with morin. Fresenius J Anal Chem. 1992;342:839–45.
Dean JH, Murray MJ. Toxic responses of the immune system. In: Amdur MO, Doull J, Klaassen CD, editors. Casarett and Doull’s toxicology: the basic science of poisons. 4th ed. New York: Pergamon Press; 1991. p. 282–333.
European Union. 2009/425/EC: Commission Decision of 28 May 2009 amending Council Directive 76/769/EEC as regards restrictions on the marketing and use of organostannic compounds for the purpose of adapting its Annex I to technical progress. In: Official Journal of the European Union, 4 June 2009; vol 4, L138/11-13. http://eur-lex.europa.en/LexUriServ/LexUriServ.do?uri=OJ:L:2009:138:0011:0013:EN:PDF. Accessed 8 Nov 2010.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kao, CH.K., Hsu, WL., Xie, HL. et al. GMP production of [18F]FDOPA and issues concerning its quality analyses as in USP “Fluorodopa F 18 Injection”. Ann Nucl Med 25, 309–316 (2011). https://doi.org/10.1007/s12149-010-0463-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-010-0463-x