Abstract
Objective
Neuroimaging plays a major role in the early diagnosis of Alzheimer’s disease (AD). Recent advances in voxelwise statistical analysis after anatomic standardization of images have made this early diagnosis easier and more objective than visual inspection. We present comparative observations of NEUROSTAT, statistical parametric mapping (SPM) 99, and SPM2 in the early diagnosis of AD using brain perfusion single-photon emission computed tomography (SPECT) and magnetic resonance imaging (MRI).
Methods
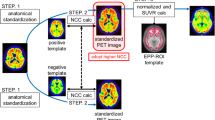
We performed voxel-by-voxel statistical group analysis for brain perfusion SPECT and gray matter images segmented from MRI between 61 patients with very early AD and 82 age-matched healthy volunteers. Anatomic standardization was performed using NEUROSTAT, SPM99, and SPM2 using both original and common templates.
Results
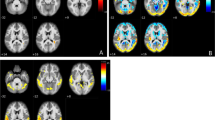
The location of significant reduction of regional cerebral blood flow (rCBF) for SPECT and gray matter concentration for MRI were identical among these three methods irrespective of the templates used. When using the original template, the significance of peak rCBF reduction in the posterior cingulate gyri was higher in SPM99 and SPM2 than that in NEUROSTAT. On the other hand, when using the common template, the significance of peak rCBF reduction in the posterior cingulate gyri was higher in NEUROSTAT and SPM2 than that in SPM99. NEUROSTAT showed almost the equal significance of peak rCBF reduction between the used templates. Almost the equal significance of reduction in gray matter concentration was observed in the parahippocampal gyri among the three methods.
Conclusions
NEUROSTAT, SPM99, and SPM2 showed identical location of significant reductions in rCBF and gray matter concentration in very early AD patients. Used templates for anatomic standardization are relevant to the results of voxelwise statistical analysis in SPM, less prominently in SPM2 than in SPM99, whereas irrelevant in NEUROSTAT.
Similar content being viewed by others
References
Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol 1997;42: 85–94.
Kogure D, Matsuda H, Ohnishi T, Asada T, Uno M, Kunihiro T, et al. Longitudinal evaluation of early Alzheimer’s disease using brain perfusion SPECT. J Nucl Med 2000;41: 1155–1162.
Chételat G, Desgranges B, De La Sayette V, Viader F, Eustache F, Baron JC. Mapping gray matter loss with voxel-based morphometry in mild cognitive impairment. Neuroreport 2002;13:1939–1943.
Hirata Y, Matsuda H, Nemoto K, Ohnishi T, Hirao K, Yamashita F, et al. Voxel-based morphometry to discriminate early Alzheimer’s disease from controls. Neurosci Lett 2005; 382:269–274.
Talairach J, Tournoux P. Co-planar stereotactic atlas of the human brain. Stuttgart: Thieme; 1998.
Frith CD, Friston KJ, Ashburner J. Principles and methods. In: Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Mazziotta JC, editors. Human brain function. San Diego: Academic Press; 1997. p. 3–159.
Minoshima S, Koeppe RA, Frey KA, Kuhl DE. Anatomic standardization: linear scaling and nonlinear warping of functional brain images. J Nucl Med 1994;35:1528–1537.
Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18- FDG PET. J Nucl Med 1995;36:1238–1248.
Hosaka K, Ishii K, Sakamoto S, Sadato N, Fukuda H, Kato T, et al. Validation of anatomical standardization of FDGPET images of normal brain: comparison of SPM and NEUROSTAT. Eur J Nucl Med Mol Imaging 2005;32:92–97.
Ishii K, Willoch F, Minoshima S, Drzezga A, Ficaro EP, Cross DJ, et al. Statistical brain mapping of 18F-FDG PET in Alzheimer’s disease: validation of anatomic standardization for atrophied brains. J Nucl Med 2001;42:548–557.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology 1984;34:939–9
Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001;58:1985–1992.
Ashburner J, Friston KJ. Voxel-based morphometry: the methods. Neuroimage 2000;11:805–821.
Ohnishi T, Matsuda H, Hashimoto T, Kunihiro T, Nishikawa M, Uema T, et al. Abnormal regional cerebral blood flow in childhood autism. Brain 2000;123:1838–1844.
Imabayashi E, Matsuda H, Asada T, Ohnishi T, Sakamoto S, Nakano S, et al. Superiority of 3-dimensional stereotactic surface projection analysis over visual inspection in discrimination of patients with very early Alzheimer’s disease from controls using brain perfusion SPECT. J Nucl Med 2004;45: 1450–1457.
Sakamoto S, Ishii K. Low cerebral glucose extraction rates in the human medial temporal cortex and cerebellum. J Neurol Sci 2000;172:41–48.
Matsuda H. Role of neuroimaging in Alzheimer’s disease, with emphasis on brain perfusion SPECT. J Nucl Med 2007; 48:1289–1300.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nishimiya, M., Matsuda, H., Imabayashi, E. et al. Comparison of SPM and NEUROSTAT in voxelwise statistical analysis of brain SPECT and MRI at the early stage of Alzheimer’s disease. Ann Nucl Med 22, 921–927 (2008). https://doi.org/10.1007/s12149-008-0211-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-008-0211-7