Abstract
Objective
Dementia with Lewy bodies (DLB) is generally characterized by a decrease in regional cerebral blood flow (rCBF) in the occipital lobe. However, not all patients with DLB have this feature. We explored characteristics of rCBF pattern changes to improve the identification of DLB, in addition to occipital hypoperfusion.
Methods
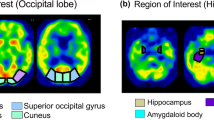
The study population comprised 30 patients with probable DLB and 49 patients with probable Alzheimer’s disease (AD) who underwent single-photon emission computed tomography. The data were analyzed using Neurological Statistical Image Analysis Software (NEUROSTAT). We established a template of the region of interest (ROI) presenting the parietal lobe, posterior cingulate, striatum, thalamus, and occipital lobe on the standard brain atlas. We then compared the mean Z scores in each ROI between DLB and AD. Moreover, we investigated the value of analyzing relative rCBF changes in both the deep gray matter and occipital lobe in differentiating DLB from AD.
Results
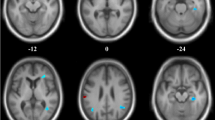
The DLB group showed a significant relative rCBF increase in the bilateral striatum and thalamus, and a significant relative rCBF decrease in the bilateral occipital lobe when compared with the AD group. Receiver-operating characteristic analysis revealed that determining the hyperperfusion in the thalamus together with the hypoperfusion in the occipital lobe enabled a more accurate differentiation between DLB and AD than studying individual areas.
Conclusions
Studying the relative increase of rCBF in the deep gray matter, and the relative decrease of that in the occipital lobe achieved a high differentiation between DLB and AD. This suggests that determining both an increase and a decrease in rCBF pattern may be important in differentiating between the two diseases.
Similar content being viewed by others
References
McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Consortium on DLB: diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872.
Ishii K, Yamaji S, Kitagaki H, Imamura T, Hirono N, Mori E. Regional cerebral blood flow difference between dementia with Lewy bodies and AD. Neurology 1999;53:413.
Lobotesis K, Fenwick JD, Phipps A, Ryman A, Swann A, Ballard C, et al. Occipital hypoperfusion on SPECT in dementia with Lewy bodies but not AD. Neurology 2001;56:643–649.
Pasquier J, Michel B, Breno-Rossi I, Hassan-Sebbag N, Sauvan R, Gastaut JL. Value of 99mTc-ECD SPET for the diagnosis of dementia with Lewy bodies. Eur J Nucl Med 2002;29:1342–1348.
Donnemiller E, Heilmann J, Wenning G, Berger W, Decristoforo C, Moncayo R, et al. Brain perfusion scintigraphy with 99mTc-HMPAO or 99mTc-ECD and 123I-β-CIT singlephoton emission tomography in dementia of the Alzheimer-type and diffuse Lewy body disease. Eur J Nucl Med 1997; 24:320–325.
Colloby SJ, Fenwick JD, Williams D, Paling SM, Lobotesis K, Ballard C, et al. A comparison of 99mTc-HMPAO SPET changes in dementia with Lewy bodies and Alzheimer’s disease using statistical parametric mapping. Eur J Nucl Med 2002;29:615–622.
Albin RL, Minosima S, D’Amato CJ, Frey KA, Kuhl DA, Sima AAF. Fluoro-deoxyglucose positron emission tomography in diffuse Lewy body disease. Neurology 1996;47:462–466.
Imamura T, Ishii K, Sasaki M, Kitagaki H, Yamaji S, Hirono N, et al. Regional cerebral glucose metabolism in dementia comparative study using positron emission tomography. Neurosci Lett 1997;235:49–52.
Ishii K, Imamura T, Sasaki M, Yamaji S, Sakamoto S, Kitagaki H, et al. Regional cerebral glucose metabolism in dementia with Lewy bodies and Alzheimer’s disease. Neurology 1998;51:125–130.
Imamura T, Ishii K, Hirono N, Hashimoto M, Tanimukai S, Kazuai H, et al. Visual hallucinations and regional cerebral metabolism in dementia with Lewy bodies (DLB). Neuroreport 1999;10:1903–1907.
Higuchi M, Tashiro M, Arai H, Okamura N, Hara S, Higuchi S, et al. Glucose hypometabolism and neuropathological correlates in brains of dementia with Lewy bodies. Exp Neurol 2000;162:247–256.
Okamura N, Arai H, Higuchi M, Tashiro M, Matsui T, Hu XS, et al. [18F]FDG-PET study in dementia with Lewy bodies and Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 2001;25:447–456.
Minoshima S, Foster NL, Sima AAF, Frey KA, Albin RL, Kuhl DE. Alzheimer’s disease versus dementia with Lewy bodies: cerebral metabolic distinction with autopsy confirmation. Ann Neurol 2001;50:358–365.
Shimizu S, Hanyu H, Kanetaka H, Iwamoto T, Koizumi K, Abe K. Differentiation of dementia with Lewy bodies from Alzheimer’s disease using brain SPECT. Dement Geriatr Cogn Disord 2005;20:25–30.
Wolfson LI, Leenders KL, Brown LL, Jones T. Alterations of regional cerebral blood flow and oxygen metabolism in Parkinson’s disease. Neurology 1985;35:1399–1405.
Imon Y, Matsuda H, Ogawa M, Kogure D, Sunohara N. SPECT image analysis using statistical parametric mapping in patients with Parkinson’s disease. J Nucl Med 1999;40: 1583–1589.
Feigin A, Antonini A, Fukuda M, De Notaris R, Benti R, Pezzoli G, et al. Tc-99m ethylene cysteinate dimer SPECT in the differential diagnosis of parkinsonism. Mov Disord 2002; 17:1265–1270.
Eidelberg D, Moeller JR, Dhawan V, Sidtis JJ, Ginos JZ, Strother SC, et al. The metabolic anatomy of Parkinson’s disease: complementary [18F] fluorodeoxyglucose and [18F] fluorodopa positron emission tomographic studies. Mov Disord 1990;5:203–213.
Eidelberg D, Moeller JR, Dhawan V, Spetsieris P, Takikawa S, Ishikawa T, et al. The metabolic topography of parkinsonism. J Cereb Blood Flow Metab 1994;14:783–801.
Eidelberg D, Edwards C, Mentis MJ, Dhawan V, Moeller JR. Movement disorders: Parkinson’s disease. In: Mazziota JC, Toga AW, Frackowiak RSJ, editors. Brain mapping: the disorders. San Diego: Academic; 2000. pp. 241–261.
Firbank MJ, Burn DJ, Mckeith IG, O’Brien JT. Longitudinal study of cerebral blood flow SPECT in Parkinson’s disease with dementia, and dementia with Lewy bodies. Int J Geriatr Psychiatry 2005;20:776–782.
O’Brien JT, Firbank MJ, Mosimann UP, Burn DJ, McKeith IG. Change in perfusion, hallucinations and fluctuations in consciousness in dementia with Lewy bodies. Psychiatry Res 2005;139:79–88.
Sato T, Hanyu H, Hirao K, Shimizu S, Kanetaka H, Iwamoto T. Deep gray matter hypoperfusion with occipital hypoperfusion in dementia with Lewy bodies. Eur J Neurol 2007;14: 1299–1301.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939–944.
Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198.
Minoshima S, Koeppe RA, Frey KA, Kuhl DE. Anatomic standardization: linear scaling and nonlinear warping of functional brain images. J Nucl Med 1994;35:1528–1537.
Talairach J, Tournoux P. Co-planar stereotactic atlas of the human brain. New York: Thieme; 1998.
Mckeith I, Mintzer J, Aarsland D, Burn D, Chiu H, Cohen-Mansfield J, et al. Dementia with Lewy bodies. Lancet Neurol 2004;3:19–28.
Knopman DS, Dekosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N, et al. Practical parameter: diagnosis of dementia (an evidence-based review), report of the quality standards subcommittee of the American Academy of neurology. Neurology 2001;56:1143–1153.
Mckeith IG, Ballard CG, Perry RH, Ince PG, O’Brien JT, Neill D, et al. Prospective validation of consensus criteria for diagnosis of dementia with Lewy bodies. Neurology 2000; 54:1050–1058.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shimizu, S., Hanyu, H., Hirao, K. et al. Value of analyzing deep gray matter and occipital lobe perfusion to differentiate dementia with Lewy bodies from Alzheimer’s disease. Ann Nucl Med 22, 911–916 (2008). https://doi.org/10.1007/s12149-008-0193-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-008-0193-5