Abstract
Objective
To determine an appropriate threshold value for delineation of the target in positron emission tomography (PET) and to investigate whether PET can delineate an internal target volume (ITV), a series of phantom studies were performed.
Methods
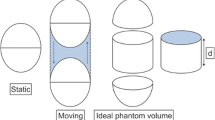
An ellipse phantom (background) was filled with 1028 Bq/ml of [18F] fluoro-2-deoxyglucose (18FDG), and six spheres of 10 mm, 13 mm, 17 mm, 22 mm, 28 mm, and 37 mm in diameter inside it were filled with 18FDG activity to achieve source-to-background (S/B) ratios of 10, 15, and 20. In static phantom experiments, an appropriate threshold value was determined so that the size of PET delineation fits to an actual sphere. In moving phantom experiments with total translations of 10 mm, 20 mm, and 30 mm and a period of oscillation of 4 s, the maximum size of PET delineation with the appropriate threshold value was measured in both the axial and sagittal planes.
Results
In the static phantom experiments, the measured maximum 18FDG activities of spheres of less than 22 mm were lower than 80% of the injected 18FDG activity, and those for the larger spheres ranged from 90% to 110%. Appropriate threshold values determined for the spheres of 22 mm or more ranged from 30% to 40% of the maximum 18FDG activity, independent of the S/B ratio. Therefore, we adopted an appropriate threshold value as 35% of the measured maximum 18FDG activity. In moving phantom experiments, the maximum 18FDG activity of spheres decreased significantly, dependent on the movement distance. Although the sizes of PET delineation with 35% threshold value tended to be slightly smaller (<3 mm) than the actual spheres in the axial plane, the longest sizes in the sagittal plane were larger than the actual spheres.
Conclusions
When a threshold value of 35% of the measured maximum 18FDG activity was adopted, the sizes of PET delineation were almost the same for static and moving phantom spheres of 22 mm or more in the axial plane. In addition, PET images have the potential to provide an individualized ITV.
Similar content being viewed by others
References
Mac Manus MP, Wong K, Hicks RJ, Matthews JP, Wirth A, Ball DL. Early mortality after radical radiotherapy for non-small-cell lung cancer: comparison of PET-staged and conventionally staged cohorts treated at a large tertiary referral center. Int J Radiat Oncol Biol Phys 2002;52:351–361.
Viney RC, Boyer MJ, King MT, Kenny PM, Pollicino CA, McLean JM, et al. Randomized controlled trial of the role of positron emission tomography in the management of stage I and II non-small-cell lung cancer. J Clin Oncol 2004;22:2357–2362.
Bradley JD, Dehdashti F, Mintun MA, Govindan R, Trinkaus K, Siegel BA. Positron emission tomography in limited-stage small-cell lung cancer: a prospective study. J Clin Oncol 2004;22:3248–3254.
Koike I, Ohmura M, Hata M, Takahashi N, Oka T, Ogino I, et al. FDG-PET scanning after radiation can predict tumor regrowth three months later. Int J Radiat Oncol Biol Phys 2003;57:1231–1238.
Lehtiö K, Eskola O, Viljanen T, Oikonen V, Grönroos T, Sillanmäki L, et al. Imaging perfusion and hypoxia with PET to predict radiotherapy response in head-and-neck cancer. Int J Radiat Oncol Biol Phys 2004;59:971–982.
Hernandez-Maraver D, Hernandez-Navarro F, Gomez-Leon N, Coya J, Rodriguez-Vigil B, Madero R, et al. Positron emission tomography/computed tomography: diagnostic accuracy in lymphoma. Br J Haematol 2006;135:293–302.
Votrubova J, Belohlavek O, Jaruskova M, Oliverius M, Lohynska R, Trskova K, et al. The role of FDG-PET/CT in the detection of recurrent colorectal cancer. Eur J Nucl Med Mol Imaging 2006;33:779–784.
Yuan S, Yu Y, Chao KS, Fu Z, Yin Y, Liu T, et al. Additional value of PET/CT over PET in assessment of locoregional lymph nodes in thoracic esophageal squamous cell cancer. J Nucl Med 2006;47:1255–1259.
Antoch G, Saoudi N, Kuehl H, Dahmen G, Mueller SP, Beyer T, et al. Accuracy of whole-body dual-modality fluorine-18-2-fluoro-2-deoxy-d-glucose positron emission tomography and computed tomography (FDG-PET/CT) for tumor staging in solid tumors: comparison with CT and PET. J Clin Oncol 2004;22:4357–4368.
Keidar Z, Haim N, Guralnik L, Wollner M, Bar-Shalom R, Ben-Nun A, et al. PET/CT using 18F-FDG in suspected lung cancer recurrence: diagnostic value and impact on patient management. J Nucl Med 2004;45:1640–1646.
Faria SL, Menard S, Devic S, Sirois C, Souhami L, Lisbona R, et al. Impact of FDG-PET on radiation therapy volume delineation in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2004;59:78–86.
Ciernik IF, Dizendorf E, Baumert BG, Reiner B, Burger C, Davis JB, et al. Radiation treatment planning with an integrated positron emission and computer tomography (PET/CT): a feasibility study. Int J Radiat Oncol Biol Phys 2003;57:853–863.
van Der Wel A, Nijsten S, Hochstenbag M, Lamers R, Boersma L, Wanders R, et al. Increased therapeutic ratio by 18FDG-PET CT planning in patients with clinical CT stage N2-N3M0 non-small-cell lung cancer: a modeling study. Int J Radiat Oncol Biol Phys 2005;61:649–655.
Heron DE, Andrade RS, Flickinger J, Johnson J, Agarwala SS, Wu A, et al. Hybrid PET-CT simulation for radiation treatment planning in head-and-neck cancers: a brief technical report. Int J Radiat Oncol Biol Phys 2004;60:1419–1424.
Vrieze O, Haustermans K, De Wever W, Lerut T, Van Cutsem E, Ectors N, et al. Is there a role for FDG-PET in radiotherapy planning in esophageal carcinoma? Radiother Oncol 2004;73:269–275.
Nishioka T, Shiga T, Shirato H, Tsukamoto E, Tsuchiya K, Kato T, et al. Image fusion between 18FDG-PET and MRI/CT for radiotherapy planning of oropharyngeal and nasopharyngeal carcinomas. Int J Radiat Oncol Biol Phys 2002;53:1051–1057.
Mutic S, Malyapa RS, Grigsby PW, Dehdashti F, Miller TR, Zoberi I, et al. PET-guided IMRT for cervical carcinoma with positive para-aortic lymph nodes—a dose-escalation treatment planning study. Int J Radiat Oncol Biol Phys 2003;55:28–35.
Caldwell CB, Mah K, Ung YC, Danjoux CE, Balogh JM, Ganguli SN, et al. Observer variation in contouring gross tumor volume in patients with poorly defined non-small cell lung tumors on CT: the impact of 18FDG-Hybrid PET fusion. Int J Radiat Oncol Biol Phys 2001;51:923–931.
Erdi YE, Rosenzweig K, Erdi AK, Macapinlac HA, Hu YC, Braban LE, et al. Radiotherapy treatment planning for patients with non-small cell lung cancer using positron emission tomography (PET). Radiother Oncol 2002;62:51–60.
Mah K, Caldwell CB, Ung YC, Danjoux CE, Balogh JM, Ganguli SN, et al. The impact of 18 FDG-PET on target and critical organs in CT-based treatment planning of patients with poorly defined non-small-cell lung carcinoma: a prospective study. Int J Radiat Oncol Biol Phys 2002;52:339–350.
Erdi YE, Mawlawi O, Larson SM, Imbriaco M, Yeung H, Finn R, et al. Segmentation of lung lesion volume by adaptive positron emission tomography image thresholding. Cancer 1997;80:2505–2509.
Nagel CC, Bosmans G, Dekker AL, Ollers MC, De Ruysscher DK, Lambin P, et al. Phased attenuation correction in respiration correlated computed tomography/positron emitted tomography. Med Phys 2006;33:1840–1847.
Caldwell CB, Mah K, Skinner M, Danjoux CE. Can PET provide the 3D extent of tumor for individualized internal target volumes? A phantom study of the limitations of CT and the promise of PET. Int J Radiat Oncol Biol Phys 2003;55:1381–1393.
Deniaud-Alexandre E, Touboul E, Lerouge D, Grahek D, Foulquier JN, Petegnief Y, et al. Impact of computed tomography and 18F-deoxyglucose coincidence detection emission tomography image fusion for optimization of conformal radiotherapy in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2005;63:1432–1441.
Ford EC, Kinahan PE, Hanlon L, Alessio A, Rajendran J, Schwartz DL, et al. Tumor delineation using PET in head and neck cancers: threshold contouring and lesion volumes. Med Phys 2006;33:4280–4288.
Nestle U, Kremp S, Schaefer-Schuler A, Sebastian-Welsch C, Hellwig D, Rübe C, et al. Comparison of different methods for delineation of 18FDG-PET-positive tissue for target volume definition in radiotherapy of patients with non-small cell lung cancer. J Nucl Med 2005;46:1342–1348.
Black QC, Grills IS, Kestin LL, Wong CY, Wong JW, Martinez AA, et al. Defining a radiotherapy target with positron emission tomography. Int J Radiat Oncol Biol Phys 2004;60:1272–1282.
Davis JB, Reiner B, Huser M, Burger C, Székely G, Ciernik IF. Assessment of 18F PET signals for automatic target volume definition in radiotherapy treatment planning. Radiother Oncol 2006;80:43–50.
Yaremko B, Riauka T, Robinson D, Murray B, Alexander A, McEwan A, et al. Thresholding in PET images of static and moving targets. Phys Med Biol 2005;50:5969–5982.
Biehl KJ, Kong FM, Dehdashti F, Jin JY, Mutic S, El Naqa I, et al. 18F-FDG PET definition of gross tumor volume for radiotherapy of non-small cell lung cancer: is a single standardized uptake value threshold approach appropriate? J Nucl Med 2006;47:1808–1812.
Hong R, Halama J, Bova D, Sethi A, Emami B. Correlation of PET standard uptake value and CT window-level threshold for target delineation in CT-based radiation treatment planning. Int J Radiat Oncol Biol Phys 2007;67:720–726.
Ashamalla H, Rafla S, Parikh K, Mokhtar B, Goswami G, Kambam S, et al. The contribution of integrated PET/CT to the evolving definition of treatment volumes in radiation treatment planning in lung cancer. Int J Radiat Oncol Biol Phys 2005;63:1016–1023.
Plathow C, Ley S, Fink C, Puderbach M, Hosch W, Schmähl A, et al. Analysis of intrathoracic tumor mobility during whole breathing cycle by dynamic MRI. Int J Radiat Oncol Biol Phys 2004;59:952–959.
Ross CS, Hussey DH, Pennington EC, Stanford W, Doornbos JF. Analysis of movement of intrathoracic neoplasms using ultrafast computerized tomography. Int J Radiat Oncol Biol Phys 1990;18:671–677.
Stevens CW, Munden RF, Forster KM, Kelly JF, Liao Z, Starkschall G, et al. Respiratory-driven lung tumor motion is independent of tumor size, tumor location, and pulmonary function. Int J Radiat Oncol Biol Phys 2001;51:62–68.
Sasaki R, Komaki R, Macapinlac H, Erasmus J, Allen P, Forster K, et al. [18F]fluorodeoxyglucose uptake by positron emission tomography predicts outcome of non-small-cell lung cancer. J Clin Oncol 2005;23:1136–1143.
Sarrut D, Boldea V, Miguet S, Ginestet C. Simulation of 4D CT Images from deformable registration between inhale and exhale breath-hold CT scans. Int J Radiat Oncol Biol Phys 2005;63:S509–S510.
Low DA, Nystrom M, Kalinin E, Parikh P, Dempsey JF, Bradley JD, et al. A method for the reconstruction of four-dimensional synchronized CT scans acquired during free breathing. Med Phys 2003;30:1254–1263.
Britton KR, Starkschall G, Tucker SL, Pan T, Nelson C, Chang JY, et al. Assessment of gross tumor volume regression and motion changes during radiotherapy for non-small-cell lung cancer as measured by four-dimensional computed tomography. Int J Radiat Oncol Biol Phys 2007;68:1036–1046.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okubo, M., Nishimura, Y., Nakamatsu, K. et al. Static and moving phantom studies for radiation treatment planning in a positron emission tomography and computed tomography (PET/CT) system. Ann Nucl Med 22, 579–586 (2008). https://doi.org/10.1007/s12149-008-0166-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-008-0166-8