Abstract
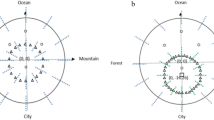
Boundary and landmark as visual spatial cues exert different effects in human spatial navigation. However, it is unclear that how different contributions between boundary and landmark are during human spatial navigation, and how their learning processes occur. In this study, we addressed these issues by using boundary-based and landmark-based spatial navigation tasks in a large sample of participants. During the task, participants were instructed to learn an object’s location based on boundary or landmark in the learning phase, and then retrieve the object’s location in the testing phase. Firstly, we found significantly lower distance and angular errors, and smaller variability during the boundary-based task than that in the landmark-based task, which suggested that boundary cue might guide more effectively and stably than landmark cue during spatial navigation. Secondly, our results showed that individual’s distance and angular errors declined less across the time in the boundary-based navigation than that in the landmark-based navigation, suggested that the boundary-based learning effect was weaker than the landmark-based learning effect. Finally, we found that boundary-influenced individuals were more proficient in the boundary-based navigation, while landmark-influenced individuals were more proficient in the landmark-based navigation. Together, these findings indicate that boundary has higher effectiveness for guiding but less enhancement for learning than landmark in spatial navigation.





Similar content being viewed by others
References
Alexander, A. S., Carstensen, L. C., Hinman, J. R., Raudies, F., Chapman, G. W., & Hasselmo, M. E. (2020). Egocentric boundary vector tuning of the retrosplenial cortex. Science Advances, 6(8), eaaz2322
Andersson, S. O., Moser, E. I., & Moser, M. B. (2021). Visual stimulus features that elicit activity in object-vector cells. Communications Biology, 4(1), 1–13
Bécu, M., Sheynikhovich, D., Tatur, G., Agathos, C. P., Bologna, L. L., Sahel, J. A., & Arleo, A. (2020). Age-related preference for geometric spatial cues during real-world navigation. Nature human behaviour, 4(1), 88–99
Berens, S. C., Bird, C. M., & Harrison, N. A. (2020). Minocycline differentially modulates human spatial memory systems. Neuropsychopharmacology : Official Publication Of The American College Of Neuropsychopharmacology, 45(13), 2162–2169
Bicanski, A., & Burgess, N. (2020). Neuronal vector coding in spatial cognition. Nature Reviews Neuroscience, 21(9), 453–470
Blaisdell, A. P., Schroeder, J. E., & Fast, C. D. (2018). Spatial integration during performance in pigeons. Behavioural processes, 154, 73–80
Buckley, M. G., Smith, A. D., & Haselgrove, M. (2015). Learned predictiveness training modulates biases towards using boundary or landmark cues during navigation. The Quarterly journal of experimental psychology, 68(6), 1183–1202
Bullens, J., Nardini, M., Doeller, C. F., Braddick, O., Postma, A., & Burgess, N. (2010). The role of landmarks and boundaries in the development of spatial memory. Developmental science, 13(1), 170–180
Chamizo, V. D., Manteiga, R. D., Rodrigo, T., & Mackintosh, N. J. (2006). Competition between landmarks in spatial learning: the role of proximity to the goal. Behavioural processes, 71(1), 59–65
Chen, D., Kunz, L., Wang, W., Zhang, H., Wang, W. X., Schulze-Bonhage, A., Reinacher, P. C., Zhou, W., Liang, S., Axmacher, N., & Wang, L. (2018). Hexadirectional modulation of theta power in human entorhinal cortex during spatial navigation. Current Biology, 28(20), 3310–3315
Cheng, K., Huttenlocher, J., & Newcombe, N. S. (2013). 25 years of research on the use of geometry in spatial reorientation: a current theoretical perspective. Psychonomic bulletin & review, 20(6), 1033–1054
Cheng, K. (1986). A purely geometric module in the rat’s spatial representation. Cognition, 23(2), 149–178
Coughlan, G., Puthusseryppady, V., Lowry, E., Gillings, R., Spiers, H., Minihane, A. M., & Hornberger, M. (2020). Test-retest reliability of spatial navigation in adults at-risk of Alzheimer’s disease.PLoS One, 15(9), e0239077
Deshmukh, S. S., & Knierim, J. J. (2013). Influence of local objects on hippocampal representations: Landmark vectors and memory. Hippocampus, 23(4), 253–267
Doeller, C. F., & Burgess, N. (2008). Distinct error-correcting and incidental learning of location relative to landmarks and boundaries. Proceedings of the National Academy of Sciences of the United States of America, 105(15), 5909–5914
Doeller, C. F., King, J. A., & Burgess, N. (2008). Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memory. Proceedings of the National Academy of Sciences of the United States of America, 105(15), 5915–5920
Epstein, R. A., & Vass, L. K. (2014). Neural systems for landmark-based wayfinding in humans. Philosophical Transactions of the Royal Society B: Biological Sciences, 369(1635), 20120533
Ferrara, K., Landau, B., & Park, S. (2019). Impaired behavioral and neural representation of scenes in Williams syndrome. Cortex; A Journal Devoted To The Study Of The Nervous System And Behavior, 121, 264–276
Ferguson, T. D., Livingstone-Lee, S. A., & Skelton, R. W. (2019). Incidental learning of allocentric and egocentric strategies by both men and women in a dual-strategy virtual Morris Water Maze. Behavioural brain research, 364, 281–295
Gallistel, C. R. (1990). The organization of learning. The MIT Press
Geerts, J. P., Chersi, F., Stachenfeld, K. L., & Burgess, N. (2020). A general model of hippocampal and dorsal striatal learning and decision making. Proceedings of the National Academy of Sciences of the United States of America, 117(49), 31427–31437
Gianni, E., De Zorzi, L., & Lee, S. A. (2018). The developing role of transparent surfaces in children’s spatial representation. Cognitive Psychology, 105, 39–52
Glöckner, F., Schuck, N. W., & Li, S. C. (2021). Differential prioritization of intramaze cue and boundary information during spatial navigation across the human lifespan. Scientific Reports, 11(1), 1–16
Gofman, X., Tocker, G., Weiss, S., Boccara, C. N., Lu, L., Moser, M. B., Morris, G., & Derdikman, D. (2019). Dissociation between Postrhinal Cortex and Downstream Parahippocampal Regions in the Representation of Egocentric Boundaries. Current Biology, 29(16), 2751–2757e4
Hägglund, M., Mørreaunet, M., Moser, M. B., & Moser, E. I. (2019). Grid-Cell Distortion along Geometric Borders. Current Biology, 29(6), 1047–1054. e3
He, Q., & Brown, T. I. (2019). Environmental Barriers Disrupt Grid-like Representations in Humans during Navigation. Current Biology, 29(16), 2718–2722. e3
Hébert, M., Bulla, J., Vivien, D., & Agin, V. (2017). Are Distal and Proximal Visual Cues Equally Important during Spatial Learning in Mice? A Pilot Study of Overshadowing in the Spatial Domain. Frontiers in behavioral neuroscience, 11, 109
Hinman, J. R., Chapman, G. W., & Hasselmo, M. E. (2019). Neuronal representation of environmental boundaries in egocentric coordinates. Nature Communications, 10(1), 2772
Honbolygó, F., Babik, A., & Török, Á. (2014, November). Location learning in virtual environments: The effect of saliency of landmarks and boundaries. In 2014 5th IEEE Conference on Cognitive Infocommunications (CogInfoCom) (pp. 595–598). IEEE
Julian, J. B., Kamps, F. S., Epstein, R. A., & Dilks, D. D. (2019). Dissociable spatial memory systems revealed by typical and atypical human development.Developmental science, 22(2), e12737
Julian, J. B., Ryan, J., Hamilton, R. H., & Epstein, R. A. (2016). The Occipital Place Area Is Causally Involved in Representing Environmental Boundaries during Navigation. Current biology, 26(8), 1104–1109
Kamps, F. S., Julian, J. B., Kubilius, J., Kanwisher, N., & Dilks, D. D. (2016). The occipital place area represents the local elements of scenes. Neuroimage, 132, 417–424
Keinath, A. T., Julian, J. B., Epstein, R. A., & Muzzio, I. A. (2017). Environmental Geometry Aligns the Hippocampal Map during Spatial Reorientation. Current Biology, 27(3), 309–317
Kessels, R. P., Van Doormaal, A., & Janzen, G. (2011). Landmark recognition in Alzheimer’s dementia: spared implicit memory for objects relevant for navigation.PloS One, 6(4), e18611
Kosaki, Y., Austen, J. M., & McGregor, A. (2013). Overshadowing of geometry learning by discrete landmarks in the water maze: effects of relative salience and relative validity of competing cues. Journal of experimental psychology: Animal behavior processes, 39(2), 126–139
Kosaki, Y., Poulter, S. L., Austen, J. M., & McGregor, A. (2015). Dorsolateral striatal lesions impair navigation based on landmark-goal vectors but facilitate spatial learning based on a “cognitive map”. Learning & memory, 22(3), 179–191
Krupic, J., Bauza, M., Burton, S., Barry, C., & O’Keefe, J. (2015). Grid cell symmetry is shaped by environmental geometry. Nature, 518(7538), 232–235
Kunz, L., Brandt, A., Reinacher, P. C., Staresina, B. P., Reifenstein, E. T., Weidemann, C. T., Herweg, N. A., Patel, A., Tsitsiklis, M., Kempter, R., Kahana, M. J., Schulze-Bonhage, A., & Jacobs, J. (2021). A neural code for egocentric spatial maps in the human medial temporal lobe. Neuron, 109(17), 2781–2796
Lee, S. A. (2017). The boundary-based view of spatial cognition: a synthesis. Current Opinion in Behavioral Sciences, 16, 58–65
Lee, S. A., Austen, J. M., Sovrano, V. A., Vallortigara, G., McGregor, A., & Lever, C. (2020). Distinct and combined responses to environmental geometry and features in a working-memory reorientation task in rats and chicks. Scientific Reports, 10(1), 7508
Lee, S. A., Ferrari, A., Vallortigara, G., & Sovrano, V. A. (2015). Boundary primacy in spatial mapping: Evidence from zebrafish (Danio rerio). Behavioural processes, 119, 116–122
Lee, S. A., Shusterman, A., & Spelke, E. S. (2006). Reorientation and landmark-guided search by young children: evidence for two systems. Psychological science, 17(7), 577–582
Lee, S. A., & Spelke, E. S. (2010). A modular geometric mechanism for reorientation in children. Cognitive psychology, 61(2), 152–176
Mackintosh, N. J. (1976). Overshadowing and stimulus intensity. Animal learning & behavior, 4(2), 186–192
Mou, W., & Zhou, R. (2013). Defining a boundary in goal localization: Infinite number of points or extended surfaces. Journal of experimental psychology: Learning memory and cognition, 39(4), 1115–1127
Negen, J., Sandri, A., Lee, S. A., & Nardini, M. (2020). Boundaries in spatial cognition: Looking like a boundary is more important than being a boundary. Journal of experimental psychology: Learning memory and cognition, 46(6), 1007–1021
Newcombe, N. S., & Ratliff, K. R. (2007). Explaining the development of spatial reorientation. The emerging spatial mind, 53–76. New York: Oxford University Press
Noack, H., Doeller, C. F., & Born, J. (2021). Sleep strengthens integration of spatial memory systems. Learning & Memory, 28(5), 162–170
Park, J., & Park, S. (2020). Coding of navigational distance and functional constraint of boundaries in the human scene-selective cortex. Journal of Neuroscience, 40(18), 3621–3630
Prados, J. (2011). Blocking and overshadowing in human geometry learning. Journal of Experimental Psychology: Animal Behavior Processes, 37(1), 121
Ratliff, K. R., & Newcombe, N. S. (2008). Reorienting when cues conflict: Evidence for an adaptive-combination view. Psychological science, 19(12), 1301–1307
Schuck, N. W., Doeller, C. F., Polk, T. A., Lindenberger, U., & Li, S. C. (2015). Human aging alters the neural computation and representation of space. Neuroimage, 117, 141–150
Sheynikhovich, D., & Arleo, A. (2010). A reinforcement learning approach to model interactions between landmarks and geometric cues during spatial learning. Brain research, 1365, 35–47
Sotelo, M. I., Alcalá, J. A., Bingman, V. P., & Muzio, R. N. (2020). On the transfer of spatial learning between geometrically different shaped environments in the terrestrial toad, Rhinella arenarum. Animal Cognition, 23(1), 5570
Stewart, S., Jeewajee, A., Wills, T. J., Burgess, N., & Lever, C. (2014). Boundary coding in the rat subiculum. Philosophical transactions of the Royal Society of London Series B Biological sciences, 369(1635), 20120514
Sun, L., Frank, S. M., Epstein, R. A., & Peter, U. T. (2021). The parahippocampal place area and hippocampus encode the spatial significance of landmark objects. Neuroimage, 236, 118081
Twyman, A., Friedman, A., & Spetch, M. L. (2007). Penetrating the geometric module: catalyzing children’s use of landmarks. Developmental psychology, 43(6), 1523–1530
van Wijngaarden, J. B., Babl, S. S., & Ito, H. T. (2020). Entorhinal-retrosplenial circuits for allocentric-egocentric transformation of boundary coding.eLife, 9, e59816
Vieites, V., Pruden, S. M., & Reeb-Sutherland, B. C. (2020). Childhood wayfinding experience explains sex and individual differences in adult wayfinding strategy and anxiety. Cognitive research: principles and implications, 5(1), 12
Weisberg, S. M., & Newcombe, N. S. (2016). How do (some) people make a cognitive map? Routes, places, and working memory. Journal of Experimental Psychology: Learning Memory and Cognition, 42(5), 768
Wilson, P. N., & Alexander, T. (2008). Blocking of spatial learning between enclosure geometry and a local landmark. Journal of Experimental Psychology: Learning Memory and Cognition, 34(6), 1369–1376
Wilson, P. N., & Alexander, T. (2010). Enclosure shape influences cue competition effects and goal location learning. The Quarterly Journal of Experimental Psychology, 63(8), 1552–1567
Zhou, R., & Mou, W. (2019a). Boundary shapes guide selection of reference points in goal localization. Attention Perception & Psychophysics, 81(7), 2482–2498
Zhou, R., & Mou, W. (2019b). The effects of cue placement on the relative dominance of boundaries and landmark arrays in goal localization. The Quarterly Journal of Experimental Psychology, 72(11), 2614–2631
Zhou, R., & Mou, W. (2016). Superior cognitive mapping through single landmark-related learning than through boundary-related learning. Journal of experimental psychology: Learning Memory and Cognition, 42(8), 1316–1323
Acknowledgements
This study was funded by the National Natural Science Foundation of China (31872786, 31861143039), the Natural Science Foundation of Hubei Province of China (2020CFB363), the MOE (Ministry of Education in China) Project of Humanities and Social Sciences(21YJC190005), the Open Research Fund of the Key Laboratory of Adolescent Cyberpsychology and Behavior (CCNU) (2019A01), and the Fundamental Research Funds for the Central Universities (2021XZZX006, CCNU22QN020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hao, X., Yuan, Z., Lin, S. et al. Different behavioral and learning effects between using boundary and landmark cues during spatial navigation. Curr Psychol 42, 23301–23312 (2023). https://doi.org/10.1007/s12144-022-03335-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12144-022-03335-0