Abstract
Background
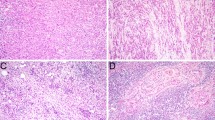
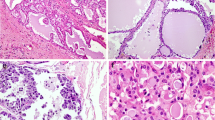
Secretory myoepithelial carcinomas (SMCA) are rare, mucinous, signet ring predominant tumors with primitive myoepithelial features. While many mucinous salivary gland tumors have now been molecularly characterized, key drivers in SMCA have yet to be elucidated. Recently, NKX3.1, a homeodomain transcription factor implicated in salivary mucous acinar development was also shown in a subset of salivary mucinous neoplasms, salivary intraductal papillary mucinous neoplasms (SG-IPMN). To date, NKX3.1 expression has not been characterized in other mucinous salivary lesions. Here, we report molecular and extended immunophenotypic findings in SMCA and NKX3.1 expression in the context of other head and neck lesions.
Methods
We retrieved 4 previously reported SMCA, performed additional immunohistochemical and targeted next-generation sequencing (NGS). We also investigated the use of NKX3.1 as a marker for SMCA in the context of its prevalence and extent (using H-score) in a mixed cohort of retrospectively and prospectively tested head and neck lesions (n = 223) and non-neoplastic tissues (n = 66).
Results
NKX3.1 positivity was confirmed in normal mucous acini as well as in mucous acinar class of lesions (5/6, mean H-score: 136.7), including mucinous adenocarcinomas (3/4), SG-IPMN (1/1), and microsecretory adenocarcinoma (MSA) (1/1). All SMCA were positive. Fluorescence in situ hybridization for SS18 rearrangements were negative in all successfully tested cases (0/3). NGS was successful in two cases (cases 3 and 4). Case 3 demonstrated a PTEN c.655C>T p.Q219* mutation and a SEC16A::NOTCH1 fusion while case 4 (clinically aggressive) showed a PTEN c.1026+1G>A p.K342 splice site variant, aTP53 c.524G>A p.R175H mutation and a higher tumor mutation burden (29 per Mb). PTEN immunohistochemical loss was confirmed in both cases and a subset of tumor cells showed strong (extreme) staining for P53 in Case 4.
Conclusion
Despite a partial myoepithelial phenotype, SMCA, along with mucinous adenocarcinomas/SG-IPMN and MSA, provisionally constitute a mucous acinar class of tumors based on morphology and NKX3.1 expression. Like salivary mucinous adenocarcinomas/SG-IPMN, SMCA also show alterations of the PTEN/PI3K/AKT pathway and may show progressive molecular alterations. We document the first extramammary tumor with a SEC16A::NOTCH1 fusion.





Similar content being viewed by others
Data Availability
Not Applicable.
Code Availability
Not Applicable.
References
Williams L, Thompson LD, Seethala RR, Weinreb I, Assaad AM, Tuluc M et al (2015) Salivary duct carcinoma: the predominance of apocrine morphology, prevalence of histologic variants, and androgen receptor expression. Am J Surg Pathol 39(5):705–713
Kusafuka K, Maeda M, Honda M, Nakajima T (2012) Mucin-rich salivary duct carcinoma with signet-ring cell feature ex pleomorphic adenoma of the submandibular gland: a case report of an unusual histology with immunohistochemical analysis and review of the literature. Med Mol Morphol 45(1):45–52
Yakirevich E, Sabo E, Klorin G, Alos L, Cardesa A, Ellis GL et al (2010) Primary mucin-producing tumours of the salivary glands: a clinicopathological and morphometric study. Histopathology 57(3):395–409
Simpson RH, Prasad AR, Lewis JE, Skalova A, David L (2003) Mucin-rich variant of salivary duct carcinoma: a clinicopathologic and immunohistochemical study of four cases. Am J Surg Pathol 27(8):1070–1079
Seethala RR, Chiosea SI (2016) MAML2 status in mucoepidermoid carcinoma can no longer be considered a prognostic marker. Am J Surg Pathol 40(8):1151–1153
Petersson F, Michal M, Kazakov DV, Grossmann P, Michal M (2016) A new hitherto unreported histopathologic manifestation of mammary analogue secretory carcinoma: “masked MASC” associated with low-grade mucinous adenocarcinoma and low-grade in situ carcinoma components. Appl Immunohistochem Mol Morphol 24(9):e80–e85
Bishop JA, Weinreb I, Swanson D, Westra WH, Qureshi HS, Sciubba J et al (2019) Microsecretory adenocarcinoma: a novel salivary gland tumor characterized by a recurrent MEF2C-SS18 fusion. Am J Surg Pathol 43(8):1023–1032
Weinreb I, Hahn E, Dickson BC, Rooper LM, Rupp NJ, Freiberger SN et al (2022) Microcribriform adenocarcinoma of salivary glands: a unique tumor entity characterized by an SS18::ZBTB7A fusion. Am J Surg Pathol. https://doi.org/10.1097/PAS.0000000000001980
Rooper LM, Argyris PP, Thompson LDR, Gagan J, Westra WH, Jordan RC et al (2021) Salivary mucinous adenocarcinoma is a histologically diverse single entity with recurrent AKT1 E17K mutations: clinicopathologic and molecular characterization with proposal for a unified classification. Am J Surg Pathol 45(10):1337–1347
Agaimy A, Mueller SK, Bumm K, Iro H, Moskalev EA, Hartmann A et al (2018) Intraductal papillary mucinous neoplasms of minor salivary glands with AKT1 p.Glu17Lys mutation. Am J Surg Pathol 42(8):1076–1082
Nakaguro M, Sadow PM, Hu R, Hattori H, Kuwabara K, Tsuzuki T et al (2022) NKX3.1 Expression in salivary gland “intraductal” papillary mucinous neoplasm: a low-grade subtype of salivary gland mucinous adenocarcinoma. Head Neck Pathol 16:1114
Ghannoum JE, Freedman PD (2004) Signet-ring cell (mucin-producing) adenocarcinomas of minor salivary glands. Am J Surg Pathol 28(1):89–93
Gnepp DR (2013) Mucinous myoepithelioma, a recently described new myoepithelioma variant. Head Neck Pathol 7(Suppl 1):S85–S89
Bastaki JM, Purgina BM, Dacic S, Seethala RR (2014) Secretory myoepithelial carcinoma: a histologic and molecular survey and a proposed nomenclature for mucin producing signet ring tumors. Head Neck Pathol 8(3):250–260
Schneider A, Brand T, Zweigerdt R, Arnold H (2000) Targeted disruption of the Nkx3.1 gene in mice results in morphogenetic defects of minor salivary glands: parallels to glandular duct morphogenesis in prostate. Mech Dev 95(1–2):163–174
Gurel B, Ali TZ, Montgomery EA, Begum S, Hicks J, Goggins M et al (2010) NKX3.1 as a marker of prostatic origin in metastatic tumors. Am J Surg Pathol 34(8):1097–1105
Yang RK, Zhao P, Lu C, Luo J, Hu R (2019) Expression pattern of androgen receptor and AR-V7 in androgen-deprivation therapy-naive salivary duct carcinomas. Hum Pathol 84:173–182
Takada N, Nishida H, Oyama Y, Kusaba T, Kadowaki H, Arakane M et al (2020) Immunohistochemical reactivity of prostate-specific markers for salivary duct carcinoma. Pathobiology 87(1):30–36
Seethala RR, Dacic S, Cieply K, Kelly LM, Nikiforova MN (2010) A reappraisal of the MECT1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol 34(8):1106–1121
Chiosea SI, Williams L, Griffith CC, Thompson LD, Weinreb I, Bauman JE et al (2015) Molecular characterization of apocrine salivary duct carcinoma. Am J Surg Pathol 39(6):744–752
Boyle DP, McArt DG, Irwin G, Wilhelm-Benartzi CS, Lioe TF, Sebastian E et al (2014) The prognostic significance of the aberrant extremes of p53 immunophenotypes in breast cancer. Histopathology 65(3):340–352
McClelland RA, Finlay P, Walker KJ, Nicholson D, Robertson JF, Blamey RW et al (1990) Automated quantitation of immunocytochemically localized estrogen receptors in human breast cancer. Cancer Res 50(12):3545–3550
Patel S, Snyderman CH, Muller SK, Agaimy A, Seethala RR (2022) Sinonasal mixed transitional epithelial-seromucinous papillary glandular neoplasms with BRAF p.V600E mutations—sinonasal analogues to the sialadenoma papilliferum family tumors. Virchows Arch. 481(4):565–574
Purgina B, Bastaki JM, Duvvuri U, Seethala RR (2015) A subset of sinonasal non-intestinal type adenocarcinomas are truly seromucinous adenocarcinomas: a morphologic and immunophenotypic assessment and description of a novel pitfall. Head Neck Pathol 9(4):436–446
Rooper LM, Thompson LDR, Gagan J, Hwang JSG, London NR, Mikula MW et al (2022) Low-grade non-intestinal-type sinonasal adenocarcinoma: a histologically distinctive but molecularly heterogeneous entity. Mod Pathol 35(9):1160–1167
Yoshida KI, Machado I, Motoi T, Parafioriti A, Lacambra M, Ichikawa H et al (2020) NKX3-1 Is a useful immunohistochemical marker of EWSR1-NFATC2 sarcoma and mesenchymal chondrosarcoma. Am J Surg Pathol 44(6):719–728
Yoshida A, Hashimoto T, Ryo E, Yoshida KI, Motoi T, Yatabe Y et al (2021) Confirmation of NKX3-1 expression in EWSR1-NFATC2 sarcoma and mesenchymal chondrosarcoma using monoclonal antibody immunohistochemistry, RT-PCR, and RNA in situ hybridization. Am J Surg Pathol 45(4):578–582
Bishop JA, Sajed DP, Weinreb I, Dickson BC, Bilodeau EA, Agaimy A et al (2021) Microsecretory adenocarcinoma of salivary glands: an expanded series of 24 cases. Head Neck Pathol 15(4):1192–1201
Stoeck A, Lejnine S, Truong A, Pan L, Wang H, Zang C et al (2014) Discovery of biomarkers predictive of GSI response in triple-negative breast cancer and adenoid cystic carcinoma. Cancer Discov 4(10):1154–1167
Robinson DR, Kalyana-Sundaram S, Wu YM, Shankar S, Cao X, Ateeq B et al (2011) Functionally recurrent rearrangements of the MAST kinase and Notch gene families in breast cancer. Nat Med 17(12):1646–1651
Edwards PA, Howarth KD (2012) Are breast cancers driven by fusion genes? Breast Cancer Res 14(2):303
Ho AS, Kannan K, Roy DM, Morris LG, Ganly I, Katabi N et al (2013) The mutational landscape of adenoid cystic carcinoma. Nat Genet 45(7):791–798
Ferrarotto R, Mitani Y, Diao L, Guijarro I, Wang J, Zweidler-McKay P et al (2017) Activating NOTCH1 mutations define a distinct subgroup of patients with adenoid cystic carcinoma who have poor prognosis, propensity to bone and liver metastasis, and potential responsiveness to Notch1 inhibitors. J Clin Oncol 35(3):352–360
de Sousa LG, Jovanovic K, Ferrarotto R (2022) Metastatic adenoid cystic carcinoma: genomic landscape and emerging treatments. Curr Treat Options Oncol 23(8):1135–1150
Altemani A, Costa AF, Montalli V, Mosqueda-Taylor A, de Almeida OP, Leon JE et al (2012) Signet-ring cell change in adenoid cystic carcinoma: a clinicopathologic and immunohistochemical study of four cases. Histopathology 62:531
Funding
Trainee Research Advancement Award (Department of Pathology at University of Pittsburgh, Pittsburgh, PA).
Author information
Authors and Affiliations
Contributions
Conceptualization: SP, RRS; Methodology: AIW, JMB, SIC, ADS, RRS; Formal analysis and investigation: SP, RRS; Writing—original draft preparation: SP; Writing—review and editing: JMB, SIC, ADS, RRS; Funding acquisition: SP, RRS; Resources: AIW, SIC, ADS, RRS; Supervision: RRS.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to Participate
This study has obtained IRB approval from (University of Pittsburgh Human Research Protection Office) and the need for informed consent was waived.
Consent for Publication
For this type of study consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Part of this study was awarded a Leon Barnes award as a poster at the USCAP 2022 Annual Meeting in Los Angeles (Abstracts from USCAP 2022: Head and Neck Pathology (833) Mod Pathol 2022; 35: 897). Dr. Seethala is currently a member of the Editorial Board for Head and Neck Pathology Journal.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patel, S., Wald, A.I., Bastaki, J.M. et al. NKX3.1 Expression and Molecular Characterization of Secretory Myoepithelial Carcinoma (SMCA): Advancing the Case for a Salivary Mucous Acinar Phenotype. Head and Neck Pathol 17, 467–478 (2023). https://doi.org/10.1007/s12105-023-01524-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-023-01524-2