Abstract
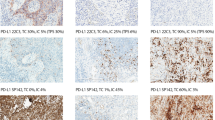
Routine testing for p16 immunohistochemistry (with selective HPV-specific test use) has been recommended for clinical practice in oropharyngeal squamous cell carcinoma (OPSCC). Data suggests that the E6H4 clone performs best for this purpose, yet no studies have evaluated the optimal antibody concentration for OPSCC testing. We evaluated three concentrations (undiluted, 1:5, and 1:10) of the primary antibody solution for E6H4 using tissue microarrays from a cohort of 199 OPSCC patients with a > 70% staining cutoff for positivity. Concordance was evaluated using percent agreement and Cohen’s kappa. The concentrations were evaluated for sensitivity and specificity using high risk HPV RNA in situ hybridization (RNA-ISH) and also correlated with Kaplan–Meier overall survival analysis. Inter-rater agreement was very high between p16 results at each concentration and also with RNA in situ hybridization (p < 0.0001 for all). Agreement between p16 undiluted and 1:5 dilution (agreement 98.2%; Kappa 0.943; p < 0.0001) was very high and between p16 undiluted and 1:10 dilution (agreement 79.2%; Kappa 0.512; p < 0.0001) much lower. Intensity of the staining did decrease with the 1:5 and 1:10 dilutions compared to undiluted, but not in a manner that obviously would change test interpretation or performance. Results suggest that the E6H4 antibody performs well at dilutions of up to 1:5 fold with a minor decrease in staining intensity, minimum loss of sensitivity, and no loss of specificity in OPSCC patients. This could result in reagent and cost savings.



Similar content being viewed by others
References
Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. New Engl J Med. 2010;363:24–35.
Gillison ML, Restighini C. Anticipation of the impact of human papillomavirus on clinical decision making for the head and neck cancer patient. Hematol/Oncol Clin North Am. 2015;29:1045–60.
Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33:3235–42.
Huang SH, Patel S, O’Sullivan B, et al. Longer survival in patients with human papillomavirus-related head and neck cancer after positive postradiation planned neck dissection. Head neck. 2015;37:946–52.
Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010;28:4142–8.
Benson E, Li R, Eisele D, Fakhry C. The clinical impact of HPV tumor status upon head and neck squamous cell carcinomas. Oral Oncol. 2014;50:565–74.
Lewis JS Jr p16 Immunohistochemistry as a standalone test for risk stratification in oropharyngeal squamous cell carcinoma. Head Neck Pathol. 2012;6(Suppl 1):S75–S82.
Sedghizadeh PP, Billington WD, Paxton D, et al. Is p16-positive oropharyngeal squamous cell carcinoma associated with favorable prognosis? A systematic review and meta-analysis. Oral Oncol. 2016;54:15–27.
Westra WH. The changing face of head and neck cancer in the 21st century: the impact of HPV on the epidemiology and pathology of oral cancer. Head Neck Pathol. 2009;3:78–81.
O’Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016;17:440–51.
O’Sullivan B, Lydiatt WM, Haughey BH, et al. HPV-Mediated (p16+) Oropharyngeal Cancer, In: Amin MB, editor. AJCC cancer staging manual. 8th edn. Cham: Springer; 2016.
Yom SS, Gillison ML, Trotti AM. Dose de-escalation in human papillomavirus-associated oropharyngeal cancer: first tracks on powder. Int J Radiat Oncol Biol Phys. 2015;93:986–8.
Shelton J, Purgina BM, Cipriani NA, et al. p16 immunohistochemistry in oropharyngeal squamous cell carcinoma: a comparison of antibody clones using patient outcomes and high-risk human papillomavirus RNA status. Mod Pathol. 2017;9:1194–203.
Lewis JS Jr, Thorstad WL, Chernock RD, et al. p16 positive oropharyngeal squamous cell carcinoma:an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol. 2010;34:1088–96.
Ukpo OC, Flanagan JJ, Ma XJ, et al. High-risk human papillomavirus E6/E7 mRNA detection by a novel in situ hybridization assay strongly correlates with p16 expression and patient outcomes in oropharyngeal squamous cell carcinoma. Am J Surg Pathol. 2011;35:1343–50.
Lewis JS Jr, Ukpo OC, Ma XJ, et al. Transcriptionally-active high-risk human papillomavirus is rare in oral cavity and laryngeal/hypopharyngeal squamous cell carcinomas—a tissue microarray study utilizing E6/E7 mRNA in situ hybridization. Histopathology. 2012;60:982–91.
Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–9.
Lewis JS Jr, Beadle B, Bishop JA, et al. (2017) Human papillomavirus testing in head and neck carcinomas—Guideline from the College of American Pathologists. Archiv Pathol Lab Med. (e-pub ahead of print).
Acknowledgements
We would like to thank Donna M. Posey for her wonderful assistance with clerical support and spreadsheet data management for the various aspects of this study. We also acknowledge the Translational Pathology Shared Resource (TPSR) supported by NCI/NIH Cancer Center Support Grant 2P30 CA068485-14 and the Vanderbilt Mouse Metabolic Phenotyping Center Grant 5U24DK059637-13.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed Consent
Use of participant data was exempt from individual consent (study waiver) under institutional review board approval.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lewis, J.S., Shelton, J., Kuhs, K.L. et al. p16 Immunohistochemistry in Oropharyngeal Squamous Cell Carcinoma Using the E6H4 Antibody Clone: A Technical Method Study for Optimal Dilution. Head and Neck Pathol 12, 440–447 (2018). https://doi.org/10.1007/s12105-017-0871-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-017-0871-5