Abstract
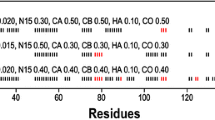
In preparation for a detailed exploration of the structural and functional aspects of the Ser2Ala mutant of human carbonic anhydrase II, we present here almost complete sequence-specific resonance assignments for 1H, 15N, and 13C. The mutation of serine to alanine at position 2, located in the N-terminal region of the enzyme, significantly alters the hydrophilic nature of the site, rendering it hydrophobic. Consequently, there is an underlying assumption that this mutation would repel water from the site. However, intriguingly, comparative analysis of the mutant structure with the wild type reveals minimal discernible differences. These assignments serve as the basis for in-depth studies on histidine dynamics, protonation states, and its intricate role in protein-water interactions and catalysis.


Similar content being viewed by others
Data availability
Assignments of Ser2Ala hCA-II has been deposited in the BMRB under accession code 52,185. Data could be shared upon request.
References
An H, Tu C, Duda D et al (2002) Chemical rescue in catalysis by human carbonic anhydrases II and III. Biochemistry 41:3235–3242
Bax A, Ikura M (1991) An efficient 3D NMR technique for correlating the proton and 15N backbone amide resonances with the alpha-carbon of the preceding residue in uniformly 15N/13C enriched proteins. J Biomol NMR 1:99–104
Bax A, Grzesiek S (1993) Methodological advances in protein NMR. Acc Chem Res 26:131–138
Chary KVR, Govil G (2008) NMR in biological systems: from molecules to human. Springer Science & Business Media, Newyork
Edison AS, Abildgaard F, Westler WM et al (1994) Practical introduction to theory and implementation of multinuclear, multidimensional nuclear magnetic resonance experiments. Methods Enzymol 239:3–79
Elder I, Han S, Tu C et al (2004) Activation of carbonic anhydrase II by active-site incorporation of histidine analogs. Arch Biochem Biophys 421:283–289
Grzesiek S, Bax A (1993) Amino acid type determination in the sequential assignment procedure of uniformly 13C/15N-enriched proteins. J Biomol NMR 3:185–204
Kay LE, Xu GY, Yamazaki T (1994) Enhanced-sensitivity triple-resonance spectroscopy with minimal H2O saturation. J Magn Reson A 109:129–133
Keeler J (2010) Understanding NMR spectroscopy. Wiley, Hoboken
Krishnamurthy VM, Kaufman GK, Urbach AR (2008) Carbonic anhydrase as a model for biophysical and physical-organic studies of proteins and protein-ligand binding. J Mol Catal A: Chem 108(3):946–1051
Liang J-Y, Lipscomb WN (1989) Theoretical study of carbonic anhydrase-catalyzed hydration of co2: a brief review. Int J Quantum Chem 36:299–312
Lindskog S, Silverman DN (2000) The catalytic mechanism of mammalian carbonic anhydrases. Springer, New York
Maupin CM, Voth GA (2010) Proton transport in carbonic anhydrase: insights from molecular simulation. Biochim Biophys Acta 1804:332–341
Maupin CM, Saunders MG, Thorpe IF et al (2008) Origins of enhanced proton transport in the Y7F mutant of human carbonic anhydrase II. J Am Chem Soc 130:11399–11408
Mikulski RL, Silverman DN (2010) Proton transfer in catalysis and the role of proton shuttles in carbonic anhydrase. Biochim Biophys Acta 1804:422–426
Muhandiram DR, Kay LE (1994) Gradient-enhanced triple-resonance three-dimensional NMR experiments with improved sensitivity. J Magn Reson B 103:203–216
Muhandiram DR, Xu G, Kay L (1993) An enhanced-sensitivity pure absorption gradient 4D 15N, 13C-edited NOESY experiment. J Biomol NMR https://doi.org/10.1007/bf00176011
Nagle JF, Morowitz HJ (1978) Molecular mechanisms for proton transport in membranes. Proc Natl Acad Sci U S A 75:298–302
Nagle JF, Tristram-Nagle S (1983) Hydrogen bonded chain mechanisms for proton conduction and proton pumping. J Membr Biol 74:1–14
Paul S, Taraphder S (2015) Determination of the reaction coordinate for a key conformational fluctuation in human carbonic anhydrase II. J Phys Chem B 119:11403–11415
Poggetti V, Salerno S, Baglini E et al (2022) Carbonic anhydrase activators for neurodegeneration: an overview. Molecules 27:2544
Roy A, Taraphder S (2006) Proton transfer pathways in the mutant His-64-Ala of human carbonic anhydrase II. Biopolymers 82:623–630
Roy A, Taraphder S (2007) Identification of proton-transfer pathways in human carbonic anhydrase II. J Phys Chem B 111:10563–10576
Schleucher J, Sattler M, Griesinger C (1993) Coherence selection by gradients without signal attenuation: application to the three-dimensional HNCO experiment. Angew Chem, Int Ed Engl 32:1489–1491
Singh H, Vasa SK, Jangra H et al (2019) Fast microsecond dynamics of the protein-water network in the active site of human carbonic anhydrase II studied by solid-state NMR spectroscopy. J Am Chem Soc 141:19276–19288
Singh H, Das CK, Vasa SK et al (2020) The active site of a prototypical “rigid” drug target is marked by extensive conformational dynamics. Angew Chem Int Ed Engl 59:22916–22921
Taraphder S, Maupin CM, Swanson JMJ, Voth GA (2016) Coupling protein dynamics with proton transport in human carbonic anhydrase II. J Phys Chem B 120:8389–8404
Tu CK, Silverman DN, Forsman C et al (1989) Role of histidine 64 in the catalytic mechanism of human carbonic anhydrase II studied with a site-specific mutant. Biochemistry 28:7913–7918
Tu C, Qian M, An H et al (2002) Kinetic analysis of multiple proton shuttles in the active site of human carbonic anhydrase. J Biol Chem 277:38870–38876
Vallurupalli P, Bouvignies G, Kay LE (2012) Studying “invisible” excited protein states in slow exchange with a major state conformation. J Am Chem Soc 134:8148–8161
Vasa SK, Singh H, Rovó P, Linser R (2018) Dynamics and interactions of a 29 kDa human enzyme studied by solid-state NMR. J Phys Chem Lett 9:1307–1311
Vasa SK, Singh H, Grohe K, Linser R (2019) Assessment of a large enzyme-drug complex by proton-detected solid-state NMR spectroscopy without deuteration. Angew Chem Int Ed Engl 58:5758–5762
Wishart DS, Sykes BD (1994) The 13C chemical-shift index: a simple method for the identification of protein secondary structure using 13C chemical-shift data. J Biomol NMR 4:171–180
Acknowledgements
We express our sincere gratitude for the invaluable support and facilities extended to us by High Field NMR at IISER Berhampur. Furthermore, we wish to extend our heartfelt appreciation to the Department of Biotechnology, New Delhi, for their generous recognition and the esteemed Ramalingaswami re-entry fellowship awarded to HS.
Funding
The Ramalingaswami re-entry fellowship, Department of Biotechnology, New Delhi, India.
Author information
Authors and Affiliations
Contributions
HS and N: Designed and conducted experiments, analyzed data, prepared figures, and authored the manuscript. N also deposited assignments in the BMRB.
Corresponding author
Ethics declarations
Conflict of interest
No competing interests are disclosed by the authors. Ethics clearance and permission to take part is not relevant.
Ethical approval
Not applicable.
Consent for publication
Each author gives their approval for it to be published.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Neelam, Singh, H. 1H, 15N and13C resonance assignments of S2A mutant of human carbonic anhydrase II. Biomol NMR Assign (2024). https://doi.org/10.1007/s12104-024-10166-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12104-024-10166-6