Abstract
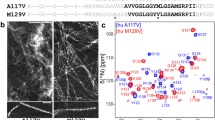
Fibrils of the protein α-synuclein (α-syn) are implicated in the pathogenesis of Parkinson’s disease and related neurodegenerative disorders. We have reported a high-resolution structure (PDB 2N0A) of an α-syn fibril form prepared by in vitro incubation of monomeric protein in 50 mM sodium phosphate buffer pH 7.4 with 0.1 mM EDTA and 0.01% sodium azide. In parallel with this structure determination, ongoing studies of small molecule ligands binding to α-syn fibrils, prepared in 2-amino-2-(hydroxymethyl)-1,3-propanediol (Tris) buffer, have been in progress, and it is therefore of interest to determine the structural similarity of these forms. Here we report the 13C and 15N resonance assignments for α-syn fibrils prepared with Tris–HCl buffer (pH 7.7 at 37 °C) and 100 mM NaCl. These fibrillization conditions yield a form with fibril core chemical shifts highly similar to those we reported (BMRB 16939) in the course of determining the high-resolution 2N0A structure, with the exception of some small perturbations from T44 to V55, including two sets of peaks observed for residues T44–V48. Additional differences occur in the patterns of observed residues in the primarily unstructured N-terminus. These results demonstrate a common fold of the fibril core for α-syn fibrils prepared in phosphate or Tris–HCl buffer at moderate ionic strength.



Similar content being viewed by others
References
Bagchi DP, Yu L, Perlmutter JS et al (2013) Binding of the radioligand SIL23 to α-synuclein fibrils in Parkinson disease brain tissue establishes feasibility and screening approaches for developing a parkinson disease imaging agent. PLoS ONE 8:e55031
Bousset L, Pieri L, Ruiz-Arlandis G et al (2013) Structural and functional characterization of two alpha-synuclein strains. Nat Commun 4:2575
Chu W, Zhou D, Gaba V et al (2015) Design, synthesis, and characterization of 3-(benzylidene)indolin-2-one derivatives as ligands for α-synuclein fibrils. J Med Chem 58:6002–6017
Comellas G, Rienstra CM (2013) Protein structure determination by magic-angle spinning solid-state NMR, and insights into the formation, structure, and stability of amyloid fibrils. Annu Rev Biophys 42:515–536
Comellas G, Lemkau LR, Nieuwkoop AJ et al (2011a) Structured regions of α-synuclein fibrils include the early-onset Parkinson’s disease mutation sites. J Mol Biol 411:881–895
Comellas G, Lopez JJ, Nieuwkoop AJ et al (2011b) Straightforward, effective calibration of SPINAL-64 decoupling results in the enhancement of sensitivity and resolution of biomolecular solid-state NMR. J Magn Reson 209:131–135
Comellas G, Lemkau LR, Zhou DH et al (2012) Structural intermediates during α-synuclein fibrillogenesis on phospholipid vesicles. J Am Chem Soc 134:5090–5099
Delaglio F, Grzesiek S, Vuister GW et al (1995) NMRPipe: a multidimensional spectral processing system based On UNIX Pipes. J Biomol NMR 6:277–293
Fung BM, Khitrin AK, Ermolaev K (2000) An improved broadband decoupling sequence for liquid crystals and solids. J Magn Reson 142:97–101
Gath J, Habenstein B, Bousset L et al (2012) Solid-state NMR sequential assignments of α-synuclein. Biomol NMR Assign 6:51–55
Gath J, Bousset L, Habenstein B et al (2014) Yet another polymorph of α-synuclein: solid-state sequential assignments. Biomol NMR Assign 8:395–404
Goddard TD, Kneller DG (2006) SPARKY 3. University of California, San Francisco
Guo JL, Covell DJ, Daniels JP et al (2013) Distinct α-synuclein strains differentially promote tau inclusions in neurons. Cell 154:103–117
Heise H, Hoyer W, Becker S et al (2005) Molecular-level secondary structure, polymorphism, and dynamics of full-length α-synuclein fibrils studied by solid-state NMR. Proc Natl Acad Sci USA 102:15871–15876
Kloepper KD, Woods WS, Winter KA et al (2006) Preparation of α-synuclein fibrils for solid-state NMR: expression, purification, and incubation of wild-type and mutant forms. Protein Expr Purif 48:112–117
Metz G, Wu X, Smith SO (1994) Ramped-amplitude cross polarization in magic-angle-spinning NMR. J Magn Reson Ser A 110:219–227
Morcombe CR, Zilm KW (2003) Chemical shift referencing in MAS solid state NMR. J Magn Reson 162:479–486
Peelaerts W, Bousset L, Van der Perren A et al (2015) α-synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522:340–344
Saborio GP, Permanne B, Soto C (2001) Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 411:810–813
Spillantini MG, Schmidt ML, Lee VM-Y et al (1997) α-Synuclein in Lewy bodies. Nature 839–840
Spillantini MG, Crowther RA, Jakes R et al (1998) α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci USA 95:6469–6473
Takegoshi K, Nakamura S, Terao T (2001) 13C–1H dipolar-assisted rotational resonance in magic-angle spinning NMR. Chem Phys Lett 344:631–637
Tuttle MD, Comellas G, Nieuwkoop AJ et al (2016a) Solid-state NMR structure of a pathogenic fibril of full-length human α-synuclein. Nat Struct Mol Biol 23:409–415
Tuttle MD, Courtney JM, Barclay AM, Rienstra CM (2016b) Protein amyloid aggregation. In: Eliezer D (ed) Methods in molecular biology (Clifton, N.J.). pp 173–183
Yu L, Cui J, Prashanth PK et al (2012) Synthesis and in vitro evaluation of α-synuclein ligands. Bioorg Med Chem 20:4625–4634
Acknowledgements
This work was supported by National Institutes of Health (NIH) Grant R01-GM073770 (to C.M.R.), a Michael J. Fox Foundation grant for nuclear magnetic resonance analysis to support development of alpha-synuclein imaging agents (to C.M.R.), and a Michael J. Fox Foundation Alpha-Synuclein Imaging Consortium grant to support development of alpha-synuclein imaging agents (to P.T.K.). A.M.B. is a trainee of the NIH Molecular Biophysics Training Grant at the University of Illinois at Urbana-Champaign (T32-GM008276). J.M.C. is a recipient of a National Science Foundation Graduate Research Fellowship. We thank Deborah A. Berthold for help with sample preparation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barclay, A.M., Dhavale, D.D., Courtney, J.M. et al. Resonance assignments of an α-synuclein fibril prepared in Tris buffer at moderate ionic strength. Biomol NMR Assign 12, 195–199 (2018). https://doi.org/10.1007/s12104-018-9808-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-018-9808-5