Abstract
Staphylococcus aureus: hibernation-promoting factor (SaHPF) is a 22.2 kDa stationary-phase protein that binds to the ribosome and turns it to the inactive form favoring survival under stress. Sequence analysis has shown that this protein is combination of two homolog proteins obtained in Escherichia coli—ribosome hibernation promoting factor (HPF) (11,000 Da) and ribosome modulation factor RMF (6500 Da). Binding site of E. coli HPF on the ribosome have been shown by X-ray study of Thermus thermophilus ribosome complex. Hence, recent studies reported that the interface is markedly different between 100S from S. aureus and E. coli. Cryo-electron microscopy structure of 100S S. aureus ribosomes reveal that the SaHPF-NTD binds to the 30S subunit as observed for shorter variants of HPF in other species and the C-terminal domain (CTD) protrudes out of each ribosome in order to mediate dimerization. SaHPF-NTD binds to the small subunit similarly to its homologs EcHPF, EcYfiA, and a plastid-specific YfiA. Furthermore, upon binding to the small subunit, the SaHPF-NTD occludes several antibiotic binding sites at the A site (hygromycin B, tetracycline), P site (edeine) and E site (pactamycin, kasugamycin). In order to elucidate the structure, dynamics and function of SaHPF-NTD from S. aureus, here we report the backbone and side chain resonance assignments for SaHPF-NTD. Analysis of the backbone chemical shifts by TALOS+ suggests that SaHPF-NTD contains two α-helices and four β-strands (β1-α1-β2-β3-β4-α2 topology). Investigating the long-term survival of S. aureus and other bacteria under antibiotic pressure could lead to advances in antibiotherapy.
Similar content being viewed by others
Biological context
Staphylococcus aureus (S. aureus) is one of the most dangerous pathogens for humans, it causes variety of community associated and nosocomial infections. Constantly increasing antimicrobial resistance of S. aureus indicates the strong requirement in development of the new drugs against this pathogen. Most of antibiotics efficiently used in clinic (more than 40%) target bacterial ribosome, a ribonucleoprotein particle synthesizing proteins in a cell. Most of them selectively inhibit translation machinery of a target bacterial cells, without disrupting the ribosomes of the host cell.
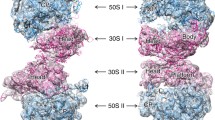
In bacteria, the protein synthesis during stress is slowed down by dimerization of the 70S ribosomes into translationally inactive 100S disomes also referred to as “hibernating ribosomes” (Yoshida and Wada 2014). This strategy allows to tune down energy consumption in the cell (Russell and Cook 1995; Szaflarski and Nierhaus 2007), to protect the ribosomes from degradation (Basu and Yap 2016) and to tolerate some antibiotics (McKay and Portnoy 2015). 100S disome formation in Escherichia coli requires two proteins: hibernation promoting factor (EcHPF, 11 kDa) and ribosome modulation factor (EcRMF, 6.5 kDa) (Ueta et al. 2005, 2008). In contrast to E. coli, S. aureus possesses only one but long two-domain protein SaHPF (22.2 kDa), which is responsible for ribosome dimerization (Ueta et al. 2010). Notably, its N-terminal domain (NTD) has high homology with EcHPF, however its C-terminal domain (CTD) has almost no homology with RMF protein (Ayupov and Akberova 2016a, b; Ueta et al. 2010). Our recent cryo electron microscopy (cryo-EM) structures of S. aureus 100S (Khusainov et al. 2017) revealed its markedly different interface in comparison to E. coli 100S structures which were reported earlier (Kato et al. 2010; Ortiz et al. 2010; Polikanov et al. 2012). It occurs mainly due to particular involvement and localization of the SaHPF-CTD, while the SaHPF-NTD was found to bind similar region as its counterpart from E. coli (Polikanov et al. 2012). Our cryo-EM reconstructions revealed that SaHPF-NTD binds to the small ribosomal subunit as observed for shorter variants of HPF and its homologs in other species (Bieri et al. 2017; Polikanov et al. 2012; Sharma et al. 2010; Vila-Sanjurjo et al. 2004). Importantly, when SaHPF-NTD is bound to the ribosome it occludes the binding of several antibiotics such as hygromycin B, tetracycline, edeine. pactamycin, kasugamycin (Borovinskaya et al. 2008; Brodersen et al. 2000; Jenner et al. 2013; Pioletti et al. 2001; Schluenzen et al. 2006). Despite similar structural organization and high sequence homology between HPF proteins, their role in stress response may be noticeably different. For instance, HPF deficient E. coli has decreased viability and stress tolerance during stationary phase (Yoshida and Wada 2014), while in B. subtilis and P. aeruginosa HPF helps to resuscitate from starvation (Akanuma et al. 2016, Akiyama et al. 2017). In S. aureus, SaHPF prevents ribosome degradation and interferes with translation initiation (Basu and Yap 2016).
In order to elucidate the structure, dynamics and function of SaHPF-NTD we have obtained its NMR assignments. Further structural studies of SaHPF-NTD will facilitate interpretation of our cryo-EM data and provide more detailed characterization of the interaction between SaHPF-NTD and the functional sites of S. aureus ribosome.
Methods and experiments
N-terminal domain of SaHPF (SaHPF-NTD, residues 1-110) protein was expressed in E. coli BL21star(DE3) cells with a pGS21A plasmid with histidine tag fused protein at its C-terminus. Uniform labeling of protein with 15N and 13C, 15N was expressed in M9 minimal medium containing 15NH4Cl and [13C6]-glucose as the sole source of nitrogen and carbon, respectively. The cells were dissolved in the lysis buffer (20 mM Tris–HCl, 500 mM NH4Cl, pH 7.6) with adding PIC and PMSF, and the carrier protein was eliminated by Ni–NTA chromatography. After a final step of gel-filtration chromatography on Superdex 75 10/300, the purified protein was dissolved in several kinds of aqueous buffer (PBS; Tris–HCl with various NH4Cl concentrations) (Ayupov et al. 2016c).
NMR spectroscopy
The NMR investigation was done using 0.62 mM sample of 13C, 15N-labeled and 15N-labeled SaHPF-NTD in PBS buffer (90%H2O + 10%D2O) at pH 7.6 with 200 mM NH4Cl concentration. NMR spectra were acquired at 35 °C on a Bruker Avance III HD 700 MHz spectrometer equipped with a quadruple resonance (1H, 13C, 15N, 31P) CryoProbe.
Sequential assignments for the backbone were obtained using the following 3D spectra: HNCO, HNCA, HN(CA)CO, HNCACB, CBCA(CO)NH, CC(CO)NH and HCC(CO)NH. Assignment of side chain resonances were obtained using the following spectra: 3D HCCH-TOCSY and 13C–1H HSQC-NOESY. Spectra were processed by TOPSPIN 3.1 (Bruker), and analyzed with Collaborative Computing Project for NMR (CCPN) (Vranken et al. 2005).
Assignments and data deposition
Chemical shift assignments were made for resonances from 108 of 110 residues (98%) of SaHPF-NTD protein. Backbone amide resonances were assigned for 96% of the non-proline residues (Fig. 1). Unassigned residues Tyr47 and Ser48 were clustered in the loop between beta-sheets β2 and β3. They remain unassigned due to fast hydrogen exchange leading to the absence of the corresponding amide resonances in the 15N–1H HSQC spectrum and 15N–1H-based heteronuclear 3D experiments. Chemical shift assignments have been obtained for 80.5% of all carbon, 70.2 of all proton and 64% of all nitrogen atoms.
Analysis of the backbone chemical shifts by TALOS+ (Shen et al. 2009) suggests that SaHPF-NTD contains two α-helices and four β-strands (β1-α1-β2-β3-β4-α2 topology), so that the α1-helix comprises residues 15–31, the α2-helix comprises residues 74–99 and the β-sheet is composed of β1 (residues 3–7), β2 (38–47), β3 (52–59), and β4 (64–70) (see Fig. 2). Position of secondary structure elements is slightly different to that observed in HPF from the E. Coli (Ueta et al. 2010). The average value of the observed correlation time τc for 15NH groups of the SaHPF-NTD was 6.55 ns which corresponds to monomer 110 a.a. protein (Daragan and Mayo 1997) (Fig. 2).
Amino acid sequence of the N-terminal domain of Staphylococcus aureus hibernation promoting factor and TALOS+ secondary structure prediction. Residues shown in gray have not been detected in 15N–1H heteronuclear NMR spectra. Correlation time τ c values for 15NH plotted versus the SaHPF-NTD residue number are identified by triangles. The cartoons represent the portions of the SaHPF-NTD domain predicted by TALOS to be α-helical (ribbon) or β-strand (arrow). Chemical shift index (CSI) consensus calculated using the CCPNMR (Berjanskii and Wishart 2005) drawn as vertical bar chart. Slowly and intermediate exchanging protons are identified by filled and empty circles, respectively
Detailed analysis of protein structure, dynamic properties and protein–ribosome interactions is a subject of ongoing studies.
The 1H, 15N and 13C chemical shift assignments have been deposited in the BioMagResBank (http://www.bmrb.wisc.edu) under the accession number BMRB-27085.
Abbreviations
- IPTG:
-
Isopropyl-thio-β-d-galactoside
- S. aureus :
-
Staphylococcus aureus
- RMF:
-
Ribosome modulation factor
- SaHPF:
-
Staphylococcus aureus hibernation promoting factor
- SaHPF-NTD:
-
N-terminal domain of SaHPF
- SaHPF-CTD:
-
C-terminal domain of SaHPF
- Cryo-EM:
-
Cryo-electron microscopy
- PIC:
-
Protease inhibitor cocktail
- PMSF:
-
Phenylmethane sulfonyl fluoride
References
Akanuma G, Kazo Y, Tagami K, Hiraoka H, Yano K, Suzuki S, Hanai R, Nanamiya H, Kato-Yamada Y, Kawamura F (2016) Ribosome dimerization is essential for the efficient regrowth of Bacillus subtilis. Microbiology 162(3):448–458
Akiyama T, Williamson KS, Schaefer R, Pratt Sh, Chang CB, Franklin MJ (2017) Resuscitation of Pseudomonas aeruginosa from dormancy requires hibernation promoting factor (PA4463) for ribosome preservation. PNAS 114:3204–3209. doi:10.1073/pnas.1700695114
Ayupov RK, Akberova NI (2016a) Prediction of the three-dimensional structure of the protein SaHPF and analysis of its molecular dynamics. Int J Pharm Technol 8:14548–14557
Ayupov RKh, Akberova NI (2016b) Analysis of the structure of SaHPF Staphylococcus aureus protein. Uchenye Zapiski Kazanskogo Universiteta Seriya Estestvennye Nauki 158:327–337
Ayupov RK, Khusainov ISh, Validov SZ, Yusupova GZ, Yusupov MM (2016c) Isolation and purification of Staphylococcus aureus hibernation promoting factor inactivating of the ribosome. Int J Pharm Technol 8:14392–143986
Basu A, Yap MNF (2016) Ribosome hibernation factor promotes Staphylococcal survival and differentially represses translation. Nucleic Acids Res 44(10):4881–4893
Berjanskii MV, Wishart DS (2005) A simple method to predict protein flexibility using secondary chemical shifts. J Am Chem Soc 127:14970–14971. doi:10.1021/ja054842f
Bieri P, Leibundgut M, Saurer M, Boehringer D, Ban N (2017) The complete structure of the chloroplast 70S ribosome in complex with translation factor pY. EMBO J 36:475–486. doi:10.15252/embj.201695959
Borovinskaya MA, Shoji S, Fredrick K, Cate JH (2008) Structural basis for hygromycin B inhibition of protein biosynthesis. RNA 14:1590–1599. doi:10.1261/rna.1076908
Brodersen DE, Clemons WM Jr, Carter AP, Morgan-Warren RJ, Wimberly BT, Ramakrishnan V (2000) The structural basis for the action of the antibiotics tetracycline, pactamycin, and hygromycin B on the 30S ribosomal subunit. Cell 103:1143–1154
Daragan VA, Mayo KH (1997) Motional model analyses of protein and peptide dynamics using 13C and 15N NMR relaxation. Prog NMR Spectrosc 31:63–105
Jenner L et al (2013) Structural basis for potent inhibitory activity of the antibiotic tigecycline during protein synthesis. Proc Nat Acad Sci USA 110:3812–3816. doi:10.1073/pnas.1216691110
Kato T, Yoshida H, Miyata T, Maki Y, Wada A, Namba K (2010) Structure of the 100S ribosome in the hibernation stage revealed by electron cryomicroscopy. Structure 18(6):719–724
Khusainov I et al (2016) Structure of the 70S ribosome from human pathogen Staphylococcus aureus. Nucl Acids Res 44:10491–10504. doi:10.1093/nar/gkw1126
Khusainov I, Vicens Q, Ayupov R, Usachev K, Myasnikov A, Simonetti A, Validov Sh, Kieffer B, Yusupova G, Yusupov M, Hashem Ya (2017) Structures and dynamics of hibernating ribosomes from S. aureus mediated by intermolecular interactions of HPF. EMBO J 36(14):2073–2087. doi:10.15252/embj.201696105
McKay SL, Portnoy DA (2015) Ribosome hibernation facilitates tolerance of stationary-phase bacteria to aminoglycosides. Antimicrob Agents Chemother 59(11):6992–6999
Ortiz JO, Brandt F, Matias VRF, Sennels L, Rappsilber J, Scheres SHW, Eibauer M, Hartl FU, Baumeister W (2010) Structure of hibernating ribosomes studied by cryoelectron tomography in vitro and in situ. J Cell Biol 190(4):613–621
Pioletti M et al (2001) Crystal structures of complexes of the small ribosomal subunit with tetracycline, edeine and IF3. EMBO J 20:1829–1839. doi:10.1093/emboj/20.8.1829
Polikanov YS, Blaha GM, Steitz TA (2012) How hibernation factors RMF, HPF, and YfiA turn off protein synthesis. Science 336:915–918. doi:10.1126/science.1218538
Russell JB, Cook GM (1995) Energetics of bacterial growth: balance of anabolic and catabolic reactions. Microbiol Rev 59:48–62
Schluenzen F et al (2006) The antibiotic kasugamycin mimics mRNA nucleotides to destabilize tRNA binding and inhibit canonical translation initiation. Nat Struct Mol Biol 13:871–878. doi:10.1038/nsmb1145
Sharma MR et al (2010) PSRP1 is not a ribosomal protein, but a ribosome-binding factor that is recycled by the ribosome-recycling factor (RRF) and elongation factor G (EF-G). J Biol Chem 285:4006–4014. doi:10.1074/jbc.M109.062299
Shen Y, Delaglio F, Cornilescu G, Bax A (2009) TALOS plus: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR 44:213–223. doi:10.1007/s10858-009-9333-z
Szaflarski W, Nierhaus KH (2007) Question 7: optimized energy consumption for protein synthesis. Origins of life and evolution of the biosphere. J Int Soc Study Orig Life 37:423–428. doi:10.1007/s11084-007-9091-4
Ueta M, Yoshida H, Wada C, Baba T, Mori H, Wada A (2005) Ribosome binding proteins YhbH and YfiA have opposite functions during 100S formation in the stationary phase of Escherichia coli. Genes Cells 10(12):1103–1112
Ueta M, Ohniwa RL, Yoshida H, Maki Y, Wada C, Wada A (2008) Role of HPF (hibernation promoting factor) in translational activity in Escherichia coli. J Biochem 143(3):425–433
Ueta M, Wada C, Wada A (2010) Formation of 100S ribosomes in Staphylococcus aureus by the hibernation promoting factor homolog SaHPF. Genes Cells 15:43–58. doi:10.1111/j.1365-2443.2009.01364.x
Vila-Sanjurjo A, Schuwirth BS, Hau CW, Cate JH (2004) Structural basis for the control of translation initiation during stress. Nat Struct Mol Biol 11:1054–1059. doi:10.1038/nsmb850
Vranken WF et al (2005) The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins 59:687–696. doi:10.1002/prot.20449
Yoshida H, Wada A (2014) The 100S ribosome: ribosomal hibernation induced by stress. Wiley Interdiscipl Rev RNA 5:723–732. doi:10.1002/wrna.1242
Acknowledgements
This work was supported by the Russian Science Foundation (Grant 16-14-10014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Usachev, K.S., Ayupov, R.K., Validov, S.Z. et al. NMR assignments of the N-terminal domain of Staphylococcus aureus hibernation promoting factor (SaHPF). Biomol NMR Assign 12, 85–89 (2018). https://doi.org/10.1007/s12104-017-9783-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-017-9783-2