Abstract
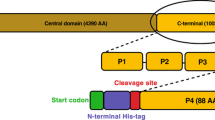
The C-terminus of the human adenosine A2A receptor differs from the other human adenosine receptors by its exceptional length and lack of a canonical cysteine residue. We have previously structurally characterized this C-terminal domain and its interaction with calmodulin. It was shown to be structurally disordered and flexible, and to bind calmodulin with high affinity in a calcium-dependent manner. Interaction with calmodulin takes place at the N-terminal end of the A2A C-terminal domain without major conformational changes in the latter. NMR was one of the biophysical methods used in the study. Here we present the HN, N, Cα, Cβ and C′ chemical shift assignments of the free form of the C-terminus residues 293–412, used in the NMR spectroscopic characterization of the domain.


Similar content being viewed by others
References
Canela L, Luján R, Lluís C, Burgueño J, Mallol J, Canela EI, Franco R, Ciruela F (2007) The neuronal Ca2+-binding protein 2 (NECAB2) interacts with the adenosine A2A receptor and modulates the cell surface expression and function of the receptor. Mol Cell Neurosci 36:1–12
Gsandtner I, Charalambous C, Stefan E, Ogris E, Freissmuth M, Zezula J (2005) Heterotrimeric G protein-independent signaling of a G protein-coupled receptor. Direct binding of ARNO/cytohesin-2 to the carboxyl terminus of the A2A adenosine receptor is necessary for sustained activation of the ERK/MAP kinase pathway. J Biol Chem 280:31898–31905
Hellman M, Piirainen H, Jaakola V-P, Permi P (2014) Bridge over troubled proline: assignment of intrinsically disordered proteins using (HCA)CON(CAN)H and (HCA)N(CA)CO(N)H experiments concomitantly with HNCO and i(HCA)CO(CA)NH. J Biomol NMR 58:49–60
Keuerleber S, Gsandtner I, Freissmuth M (2011) From cradle to twilight: the carboxyl terminus directs the fate of the A2A-adenosine receptor. Biochim Biophys Acta 1808:1350–1357
Mäntylahti S, Tossavainen H, Hellman M, Permi P (2009) An intraresidual i(HCA)CO(CA)NH experiment for the assignment of main-chain resonances in 15N, 13C labeled proteins. J Biomol NMR 45:301–310
Permi P, Annila A (2004) Coherence transfer in proteins. Prog Nucl Magn Reson Spectr 44:97–137
Piirainen H, Hellman M, Tossavainen H, Permi P, Kursula P, Jaakola V-P (2015) Human adenosine A2A receptor binds calmodulin with high affinity in a calcium-dependent manner. Biophys J 108:903–917
Sun C-N, Cheng H-C, Chou J-I, Lee S-Y, Lin Y-W, Lai H-L, Chen H-M, Chern Y (2006) Rescue of p53 blockage by the A2A adenosine receptor via a novel interacting protein, translin-associated protein X. Mol Pharmacol 70:454–466
Woods AS, Marcellino D, Jackson SN, Franco R, Ferré S, Agnati LF, Fuxe K (2008) How calmodulin interacts with the adenosine A2A and the dopamine D2 receptors. J Proteome Res 7:3428–3434
Acknowledgments
This work was supported by the Academy of Finland Grant 259447 to PP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tossavainen, H., Hellman, M., Piirainen, H. et al. HN, N, Cα, Cβ and C′ assignments of the intrinsically disordered C-terminus of human adenosine A2A receptor. Biomol NMR Assign 9, 403–406 (2015). https://doi.org/10.1007/s12104-015-9618-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-015-9618-y