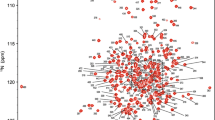
Abstract
Phosphoethanolamine methyltransferases (PMTs also known as PEAMTs) catalyze the three-step s-adenosyl-methionione-dependent methylation of phosphoethanolamine to form phosphocholine. These enzymes play an important function in the synthesis of phosphatidylcholine, the major phospholipid in the membranes of lower and higher eukaryotes, as well as in the production of the compatible solute and osmoprotectant glycine betaine in plants. Genetic studies in plants, Caenhorhabditis elegans and Plasmodium falciparum have demonstrated that disruption of PMT activity results in severe defects in important cellular processes such as development, replication, survival and sexual maturation and differentiation. Here we report chemical shift assignments for PfPMT, the PMT from Plasmodium falciparum. X-ray crystal structures have been recently reported for complexes of PfPMT, but the structure of the apoenzyme remains unknown. The solution structure of the apoenzyme will help to elucidate important details of the mechanism of substrate binding by PfPMT, as residues comprising the substrate binding site are inaccessible to solvent in the conformation evident in the available crystal structures. In addition to enabling determination of the solution structure of the apoenzyme, the assignments will facilitate additional investigations into the interaction of PfPMT with its substrates and inhibitors.


Similar content being viewed by others
References
Bobenchik AM, Augagneur Y, Hao B, Hoch JC, Ben Mamoun C (2011) Phosphoethanolamine methyltransferases in phosphocholine biosynthesis: functions and potential for antiparasite therapy. FEMS Microbiol Rev 35(4):609–619
Kay LE (1995) Pulsed field gradient multi-dimensional NMR methods for the study of protein structure and dynamics in solution. Prog Biophys Mol Biol 63(3):277–299
Lee SG, Kim Y, Alpert TD, Nagata A, Jez JM (2012) Structure and reaction mechanism of phosphoethanolamine methyltransferase from the malaria parasite Plasmodium falciparum: an anti-parasitic drug target. J Biol Chem 287(2):1426–1434
Pessi G, Kociubinski G, Mamoun CB (2004) A pathway for phosphatidylcholine biosynthesis in Plasmodium falciparum involving phosphoethanolamine methylation. Proc Natl Acad Sci USA 101:6206–6211
Reynolds JM, Takebe S, Choi JY, El Bissati K, Witola WH, Bobenchik AM, Hoch JC, Voelker DR, Mamoun CB (2008) Biochemical and genetic analysis of the phosphoethanolamine methyltransferase of the human malaria parasite Plasmodium falciparum. J Biol Chem 283(12):7894–7900
Shen Y, Delaglio F, Cornilescu G, Bax A (2009) TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR 44(4):213–223
Weber DJ, Gittis AG, Mullen GP, Abeygunawardana C, Lattman EE, Mildvan AS (1992) NMR docking of a substrate into the X-ray structure of staphylococcal nuclease. Proteins 13(4):275–287
Wishart DS, Sykes BD (1994) The 13C chemical-shift index: a simple method for the identification of protein secondary structure using 13C chemical-shift data. J Biomol NMR 4(2):171–180
Witola WH, El Bissati K, Pessi G, Xie C, Roepe PD, Mamoun CB (2008) Disruption of the Plasmodium falciparum PfPMT gene results in a complete loss of phosphatidylcholine biosynthesis via the serine-decarboxylase-phosphoethanolamine-methyltransferase pathway and severe growth and survival defects. J Biol Chem 283(41):27636–27643
Acknowledgments
Support from the US National Institutes of Health (grants GM47467 to J.C.H., AI51507 to C.B.M, GM092369 to S.K. Weller), the US Department of Defense (grant PR033005 to C.B.M.), the United States Department of Agriculture (grant NIFA-2010-38505-21257 to J.C.H.) and the Burroughs Wellcome Fund (grant 1006267 to C.B.M) is gratefully acknowledged. The 800 MHz NMR spectrometer at UCHC was purchased with support from NIH grant RR023041. The 900 MHz NMR spectrometer at the MIT/Harvard Center for Magnetic Resonance is supported by NIH grant EB002026. We thank Ms. Li Luo for technical assistance.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bezsonova, I., Rujan, I., Bobenchik, A.M. et al. 1H, 13C, and 15N chemical shift assignments for PfPMT, a phosphoethanolamine methyltransferase from Plasmodium falciparum . Biomol NMR Assign 7, 17–20 (2013). https://doi.org/10.1007/s12104-012-9372-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-012-9372-3