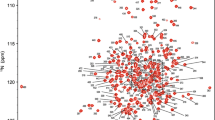
Abstract
Based on sequence homology, desulfothioredoxin (DTrx) from Desulfovibrio vulgaris Hildenborough has been identified as a new member of the thioredoxin superfamily. Desulfothioredoxin (104 amino acids) contains a particular active site consensus sequence, CPHC probably correlated to the anaerobic metabolism of these bacteria. We report the full 1H, 13C and 15N resonance assignments of the reduced and the oxidized form of desulfothioredoxin (DTrx). 2D and 3D heteronuclear NMR experiments were performed using uniformly 15N-, 13C-labelled DTrx. More than 98% backbone and 96% side-chain 1H, 13C and 15N resonance assignments were obtained. (BMRB deposits with accession number 16712 and 16713).


Similar content being viewed by others
References
Arnér ES, Holmgren A (2000) Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem/FEBS 267:6102–6109
Carvalho AP, Fernandes PA, Ramos MJ (2006) Similarities and differences in the thioredoxin superfamily. Prog Biophys Mol Biol 91:229–248
Grzesiek S, Bax A, Clore GM, Gronenborn AM, Hu JS, Kaufman J, Palmer I, Stahl SJ, Wingfield PT (1996) The solution structure of HIV-1 Nef reveals an unexpected fold and permits delineation of the binding surface for the SH3 domain of Hck tyrosine protein kinase. Nat Struct Biol 3:340–345
Holmgren A (1985) Thioredoxin. Annu Rev Biochem 54:237–271
Keller R (2004) The computer aided resonance assignment tutorial
Martin JL (1995) Thioredoxin—a fold for all reasons. Structure (London, England: 1993) 3:245–250
Pereira PM, He Q, Xavier AV, Zhou J, Pereira IAC, Louro RO (2008) Transcriptional response of Desulfovibrio vulgaris Hildenborough to oxidative stress mimicking environmental conditions. Arch Microbiol 189:451–461
Vita N, Hatchikian EC, Nouailler M, Dolla A, Pieulle L (2008) Disulfide bond-dependent mechanism of protection against oxidative stress in pyruvate-ferredoxin oxidoreductase of anaerobic Desulfovibrio bacteria. Biochemistry 47:957–964
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garcin, E.B., Bornet, O., Pieulle, L. et al. 1H, 13C and 15N backbone and side-chain chemical shift assignments for oxidized and reduced desulfothioredoxin. Biomol NMR Assign 4, 135–137 (2010). https://doi.org/10.1007/s12104-010-9226-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-010-9226-9