Abstract
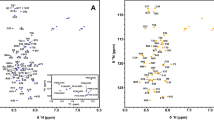
Imatinib (Glivec or Gleevec) potently inhibits the tyrosine kinase activity of BCR-ABL, a constitutively activated kinase, which causes chronic myelogenous leukemia (CML). Here we report the first almost complete backbone assignment of c-ABL kinase domain in complex with imatinib.

Similar content being viewed by others
References
Cowan-Jacob SW, Fendrich G, Floersheimer A, Furet P, Liebetanz J, Rummel G, Rheinberger P, Centeleghe M, Fabbro D, Manley PW (2007) Structural biology contributions to the discovery of drugs to treat chronic myelogenous leukaemia. Acta Crystallogr D Biol Crystallogr 63:80–93
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe – a multidimensional spectral processing system based on unix pipes. J Biomol NMR 6:277–293
Greenman C, Stephens P, Smith R et al (2007) Patterns of somatic mutation in human cancer genomes. Nature 446:153–158
Grzesiek S, Bax A (1992) Improved 3D triple-resonance NMR techniques applied to a 31-kDa protein. J Magn Reson 96:432–440
Johnson BA, Blevins RA (1994) NMR View – a computer-program for the visualization and analysis of NMR data. J Biomol NMR 4:603–614
Nagar B, Bornmann WG, Pellicena P, Schindler T, Veach D R, Miller WT, Clarkson B, Kuriyan J (2002) Crystal structures of the kinase domain of c-Abl in complex with the small molecule inhibitors PD173955 and imatinib (STI-571). Cancer Res 62:4236–4243
Pervushin K, Riek R, Wider G, Wuthrich K (1997) Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc Natl Acad Sci USA 94:12366–12371
Ren R (2005) Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer 5:172–183
Strauss A, Bitsch F, Cutting B, Fendrich G, Graff P, Liebetanz J, Zurini M, Jahnke W (2003) Amino-acid-type selective isotope labeling of proteins expressed in Baculovirus-infected insect cells useful for NMR studies. J Biomol NMR 26:367–372
Strauss A, Bitsch F, Fendrich G, Graff P, Knecht R, Meyhack B, Jahnke W (2005) Efficient uniform isotope labeling of Abl kinase expressed in Baculovirus-infected insect cells. J Biomol NMR 31:343–349
Acknowledgements
We would like to thank Drs. Sonja Alexandra Dames and Martin Allan for their help during the initial phase of the project. This work was supported by SNF grant 31-109712 (S.G.) and Novartis Pharma AG.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material. Table containing the list of specifically labeled samples used for the assignment.
Rights and permissions
About this article
Cite this article
Vajpai, N., Strauss, A., Fendrich, G. et al. Backbone NMR resonance assignment of the Abelson kinase domain in complex with imatinib. Biomol NMR Assign 2, 41–42 (2008). https://doi.org/10.1007/s12104-008-9079-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-008-9079-7