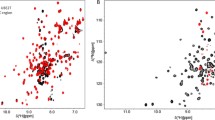
Abstract
UbcH7 is a human E2 conjugating enzyme in the ubiquitin-dependent protein degradation pathway. The resonance assignments of UbcH7 will assist in elucidating the structural basis of interactions that occur within ubiquitination.

Similar content being viewed by others
Abbreviations
- RING:
-
Really interesting new gene
- HHARI:
-
Human homologue of Drosophila ariadne
- NMR:
-
Nuclear magnetic resonance
- HSQC:
-
Heteronuclear single quantum correlation
References
Ardley HC, Tan NG, Rose SA, Markham AF, Robinson PA (2001) Features of the parkin/ariadne-like ubiquitin ligase, HHARI, that regulate its interaction with the ubiquitin-conjugating enzyme, UbcH7. J Biol Chem 276:19640–19647
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293
Grzesiek S, Bax A (1992) An efficient experiment for sequential backbone assignment of medium-sized isotopically enriched proteins. J Magn Reson 99:201–207
Hershko A, Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67:425–479
Jentsch S (1992) The ubiquitin-conjugation system. Annu Rev Genet 26:177–205
Johnson BA, Blevins RA (1994) NMRView: a computer program for the visualization and analysis of NMR data. J Biomol NMR 4:603–614
Kay LE, Keifer P, Saarinen T (1992) Pure absorption gradient enhanced heteronuclear single quantum correlation spectroscopy with improved sensitivity. J Am Chem Soc 114:10663–10665
Kay LE, Xu G, Singer AU, Muhandiram DR, Forman-Kay JD (1993) A gradient-enhanced HCCH-TOCSY experiment for recording side-chain 1H and 13C correlations in H2O samples of proteins. J Magn Reson 101:333–337
Schubert M, Oschkinat H, Schmieder P (2001) MUSIC, selective pulses, and tuned delays: amino acid type-selective 1H–15N correlations, II. J Magn Reson 148:61–72
Zheng N, Wang P, Jeffrey PD, Pavletich NP (2000) Structure of a c-Cbl-UbcH7 complex: RING domain function in ubiquitin-protein ligases. Cell 102:533–539
Acknowledgments
The authors wish to thank Dr. Phillip Robinson and Dr. Helen Ardley for the UbcH7 plasmid. We would also like to thank Kathryn Barber for her technical support and Dr. Matthew Revington for his assistance in NMR data processing. This work was supported by grants from the Canadian Institutes of Health Research (CIHR), the Canada Research Chairs Program (G.S.S.), the CIHR Canada Graduate Scholarship (S.A.S.) and the Ontario Graduate Scholarship (S.A.S.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Serniwka, S.A., Shaw, G.S. 1H, 13C and 15N resonance assignments for the human E2 conjugating enzyme, UbcH7. Biomol NMR Assign 2, 21–23 (2008). https://doi.org/10.1007/s12104-007-9074-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-007-9074-4