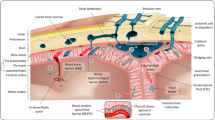
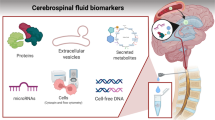
Abstract
The survival of patients with acute lymphoblastic leukemia (ALL) has dramatically improved during the last six decades. This improvement is secondary to improved diagnostics, risk stratification of treatment by biological features and response to treatment, improved supportive care, and the introduction of new treatment modalities such as immunotherapy and molecular targeted therapy. However, many questions remain concerning the involvement of the central nervous system (CNS) in leukemia, including ones pertaining to the risk factors for CNS involvement and relapse, the optimal treatment strategy to prevent relapse, and the role of newer therapies. This review discusses these questions by addressing the diagnosis of CNS leukemia, the current clinical trial data for treatment regimens with CNS activity, and issues specific to treatment in low- and middle-income countries (LMICs).

Similar content being viewed by others
References
Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373:1541–52.
Inaba H, Pui C-H. Advances in the diagnosis and treatment of pediatric acute lymphoblastic leukemia. J Clin Med. 2021;10:1926.
Arora RS, Arora B. Acute leukemia in children: a review of the current Indian data. South Asian J Cancer. 2016;5:155–60.
Thastrup M, Duguid A, Mirian C, Schmiegelow K, Halsey C. Central nervous system involvement in childhood acute lymphoblastic leukemia: challenges and solutions. Leukemia. 2022;36:2751–68.
Winick N, Devidas M, Chen S, et al. Impact of initial CSF findings on outcome among patients with National Cancer Institute standard- and high-risk B-cell acute lymphoblastic leukemia: a report from the Children’s Oncology Group. J Clin Oncol. 2017;35:2527–34.
Gossai NP, Devidas M, Chen Z, et al. Central nervous system status is prognostic in T-cell acute lymphoblastic leukemia: a Children’s Oncology Group report. Blood. 2023;141:1802–11.
Thastrup M, Marquart HV, Levinsen M, et al. Flow cytometric detection of leukemic blasts in cerebrospinal fluid predicts risk of relapse in childhood acute lymphoblastic leukemia: a nordic Society of Pediatric Hematology and Oncology study. Leukemia. 2020;34:336–46.
Gajjar A, Harrison PL, Sandlund JT, et al. Traumatic lumbar puncture at diagnosis adversely affects outcome in childhood acute lymphoblastic leukemia. Blood. 2000;96:3381–4.
Pui C-H, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9:257–68.
Howard SC, Gajjar AJ, Cheng C, et al. Risk factors for traumatic and bloody lumbar puncture in children with acute lymphoblastic leukemia. JAMA. 2002;288:2001–7.
Tang J, Yu J, Cai J, et al. Prognostic factors for CNS control in children with acute lymphoblastic leukemia treated without cranial irradiation. Blood. 2021;138:331–43.
Aur RJ, Simone J, Hustu HO, et al. Central nervous system therapy and combination chemotherapy of childhood lymphocytic leukemia. Blood. 1971;37:272–81.
Pui C-H, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–41.
Jeha S, Pei D, Raimondi SC, et al. Increased risk for CNS relapse in pre-B cell leukemia with the t(1;19)/TCF3-PBX1. Leukemia. 2009;23:1406–9.
Veerman AJ, Kamps WA, van den Berg H, et al. Dexamethasone-based therapy for childhood acute lymphoblastic leukaemia: results of the prospective dutch childhood Oncology Group (DCOG) protocol ALL-9 (1997–2004). Lancet Oncol. 2009;10:957–66.
Jeha S, Pei D, Choi J, et al. Improved CNS control of childhood acute lymphoblastic leukemia without cranial irradiation: St Jude Total Therapy Study 16. J Clin Oncol. 2019;37:3377–91.
Vora A, Andreano A, Pui C-H, et al. Influence of cranial radiotherapy on outcome in children with acute lymphoblastic leukemia treated with contemporary therapy. J Clin Oncol. 2016;34:919–26.
Matloub Y, Lindemulder S, Gaynon PS, et al. Intrathecal triple therapy decreases central nervous system relapse but fails to improve event-free survival when compared with intrathecal methotrexate: results of the Children’s Cancer Group (CCG) 1952 study for standard-risk acute lymphoblastic leukemia, reported by the Children’s Oncology Group. Blood. 2006;108:1165–73.
Salzer WL, Burke MJ, Devidas M, et al. Impact of intrathecal triple therapy versus intrathecal methotrexate on disease-free survival for high-risk B-lymphoblastic leukemia: Children’s Oncology Group Study AALL1131. J Clin Oncol. 2020;38:2628–38.
Brennan RC, Helton KJ, Pei D, et al. Spinal epidural lipomatosis in children with hematologic malignancies. Ann Hematol. 2011;90:1067–74.
Wilson R, Osborne C, Halsey C. The use of Ommaya reservoirs to deliver central nervous system-directed chemotherapy in childhood acute lymphoblastic leukaemia. Paediatr Drugs. 2018;20:293–301.
Jacola LM, Krull KR, Pui C-H, et al. Longitudinal assessment of neurocognitive outcomes in survivors of childhood acute lymphoblastic leukemia treated on a contemporary chemotherapy protocol. J Clin Oncol. 2016;34:1239–47.
Jacola LM, Conklin HM, Krull KR, et al. The impact of intensified CNS-directed therapy on neurocognitive outcomes in survivors of childhood acute lymphoblastic leukemia treated without cranial irradiation. J Clin Oncol. 2022;40:4218–27.
Banerjee P, Rossi MG, Anghelescu DL, et al. Association between anesthesia exposure and neurocognitive and neuroimaging outcomes in long-term survivors of childhood acute lymphoblastic leukemia. JAMA Oncol. 2019;5:1456–63.
Balis FM, Lester CM, Chrousos GP, Heideman RL, Poplack DG. Differences in cerebrospinal fluid penetration of corticosteroids: possible relationship to the prevention of meningeal leukemia. J Clin Oncol. 1987;5:202–7.
Bostrom BC, Sensel MR, Sather HN, et al. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: a report from the Children’s Cancer Group. Blood. 2003;101:3809–17.
Mitchell CD, Richards SM, Kinsey SE, Lilleyman J, Vora A, Eden TO. Benefit of dexamethasone compared with prednisolone for childhood acute lymphoblastic leukaemia: results of the UK Medical Research Council ALL97 randomized trial. Br J Haematol. 2005;129:734–45.
Möricke A, Zimmermann M, Valsecchi MG, et al. Dexamethasone vs prednisone in induction treatment of pediatric ALL: results of the randomized trial AIEOP-BFM ALL 2000. Blood. 2016;127:2101–12.
Vrooman LM, Neuberg DS, Stevenson KE, Supko JG, Sallan SE, Silverman LB. Dexamethasone and individualized asparaginase dosing are each associated with superior event-free survival in childhood acute lymphoblastic leukemia: results from DFCI-ALL Consortium Protocol 00–01 [abstract]. Blood. 2009;114:321.
Inaba H, Pui C-H. Glucocorticoid use in acute lymphoblastic leukaemia. Lancet Oncol. 2010;11:1096–106.
Mantadakis E, Cole PD, Kamen BA. High-dose methotrexate in acute lymphoblastic leukemia: where is the evidence for its continued use? Pharmacotherapy. 2005;25:748–55.
Larsen EC, Devidas M, Chen S, et al. Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: a report from Children’s Oncology Group Study AALL0232. J Clin Oncol. 2016;34:2380–8.
Winter SS, Dunsmore KP, Devidas M, et al. Improved survival for children and young adults with T-lineage acute lymphoblastic leukemia: results from the Children’s Oncology Group AALL0434 methotrexate randomization. J Clin Oncol. 2018;36:2926–34.
Matloub Y, Bostrom BC, Hunger SP, et al. Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Blood. 2011;118:243–51.
Woo MH, Hak LJ, Storm MC, et al. Cerebrospinal fluid asparagine concentrations after Escherichia coli asparaginase in children with acute lymphoblastic leukemia. J Clin Oncol. 1999;17:1568–73.
Rizzari C, Lanvers-Kaminsky C, Valsecchi MG, et al. Asparagine levels in the cerebrospinal fluid of children with acute lymphoblastic leukemia treated with pegylated-asparaginase in the induction phase of the AIEOP-BFM ALL 2009 study. Haematologica. 2019;104:1812–21.
Kawedia JD, Liu C, Pei D, et al. Dexamethasone exposure and asparaginase antibodies affect relapse risk in acute lymphoblastic leukemia. Blood. 2012;119:1658–64.
Gottschalk Højfeldt S, Grell K, Abrahamsson J, et al. Relapse risk following truncation of pegylated asparaginase in childhood acute lymphoblastic leukemia. Blood. 2021;137:2373–82.
Gupta S, Wang C, Raetz EA, et al. Impact of asparaginase discontinuation on outcome in childhood acute lymphoblastic leukemia: a report from the children’s Oncology Group. J Clin Oncol. 2020;38:1897–905.
Moghrabi A, Levy DE, Asselin B, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95 – 01 for children with acute lymphoblastic leukemia. Blood. 2007;109:896–904.
Berg SL, Blaney SM, Devidas M, et al. Phase II study of nelarabine (compound 506U78) in children and young adults with refractory T-cell malignancies: a report from the Children’s Oncology Group. J Clin Oncol. 2005;23:3376–82.
Kurtzberg J, Ernst TJ, Keating MJ, et al. Phase I study of 506U78 administered on a consecutive 5-day schedule in children and adults with refractory hematologic malignancies. J Clin Oncol. 2005;23:3396–403.
Dunsmore KP, Winter SS, Devidas M, et al. Children’s Oncology Group AALL0434: a phase III randomized clinical trial testing nelarabine in newly diagnosed T-cell acute lymphoblastic leukemia. J Clin Oncol. 2020;38:3282–93.
Jabbour E, O’Brien S, Kantarjian H, et al. Neurologic complications associated with intrathecal liposomal cytarabine given prophylactically in combination with high-dose methotrexate and cytarabine to patients with acute lymphocytic leukemia. Blood. 2007;109:3214–8.
Barredo JC, Devidas M, Lauer SJ, et al. Isolated CNS relapse of acute lymphoblastic leukemia treated with intensive systemic chemotherapy and delayed CNS radiation: a Pediatric Oncology Group study. J Clin Oncol. 2006;24:3142–9.
Shen S, Chen X, Cai J, et al. Effect of dasatinib vs imatinib in the treatment of pediatric Philadelphia chromosome–positive acute lymphoblastic leukemia: a randomized clinical trial. JAMA Oncol. 2020;6:358–66.
Hunger SP, Raetz EA. How I treat relapsed acute lymphoblastic leukemia in the pediatric population. Blood. 2020;136:1803–12.
Nguyen K, Devidas M, Cheng S-C, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children’s Oncology Group study. Leukemia. 2008;22:2142–50.
Harker-Murray PD, Thomas AJ, Wagner JE et al. Allogeneic hematopoietic cell transplantation in children with relapsed acute lymphoblastic leukemia isolated to the central nervous system. Biol Blood Marrow Transplant. 2008;14:685–92.
Hastings C, Chen Y, Devidas M, et al. Late isolated central nervous system relapse in childhood B-cell acute lymphoblastic leukemia treated with intensified systemic therapy and delayed reduced dose cranial radiation: a report from the Children’s Oncology Group study AALL02P2. Pediatr Blood Cancer. 2021;68:e29256.
Brown PA, Ji L, Xu X, et al. Effect of postreinduction therapy consolidation with blinatumomab vs chemotherapy on disease-free survival in children, adolescents, and young adults with first relapse of B-cell acute lymphoblastic leukemia: a randomized clinical trial. JAMA. 2021;325:833–42.
Hogan LE, Brown PA, Ji L, et al. Children's Oncology Group AALL1331: phase III trial of blinatumomab in children, adolescents, and young adults with low-risk B-cell ALL in first relapse. J Clin Oncol. 2023; online ahead of print. https://doi.org/10.1200/JCO.22.02200.2023.
Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48.
Barz Leahy A, Newman H, Li Y, et al. CD19-targeted chimeric antigen receptor T-cell therapy for CNS relapsed or refractory acute lymphocytic leukaemia: a post-hoc analysis of pooled data from five clinical trials. Lancet Haematol. 2021;8:e711–22.
Jacoby E, Ghorashian S, Vormoor B, et al. CD19 CAR T-cells for pediatric relapsed acute lymphoblastic leukemia with active CNS involvement: a retrospective international study. Leukemia. 2022;36:1525–32.
Wang T, Tang Y, Cai J, et al. Coadministration of CD19- and CD22-directed chimeric antigen receptor T-cell therapy in childhood B-cell acute lymphoblastic leukemia: a single-arm, multicenter, phase II trial. J Clin Oncol. 2023;41:1670–83.
Relling MV, Rubnitz JE, Rivera GK, et al. High incidence of secondary brain tumours after radiotherapy and antimetabolites. Lancet. 1999;354:34–9.
Acknowledgements
The authors thank Keith A. Laycock, PhD, ELS, for scientific editing of the manuscript.
Funding
This work was supported by the National Cancer Institute (Cancer Center Support CORE Grant CA21765) (S.S. and H.I.) and by the American Lebanese Syrian Associated Charities (ALSAC) (S.S. and H.I). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
SS and HI equally contributed to the writing of the manuscript; HI also provided guidance to the writing of the manuscript. HI will act as the guarantor for this manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Suryaprakash, S., Inaba, H. Acute Lymphoblastic Leukemia with Central Nervous System Involvement—Challenges in Management. Indian J Pediatr 91, 59–66 (2024). https://doi.org/10.1007/s12098-023-04731-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-023-04731-5