Abstract
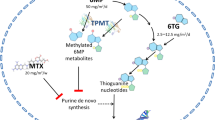
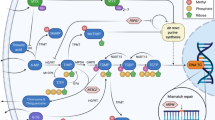
Cure rates in pediatric acute lymphoblastic leukemia (ALL) currently approach 90% in the developed world. Treatment involves 6-8 mo of intensive multi-drug chemotherapy followed by 24 mo of maintenance treatment (ALL-MT). The cornerstone of ALL-MT is the daily administration of oral 6-mercaptopurine (6MP), a purine analogue. 6MP is combined with weekly oral methotrexate (MTX), an antifolate drug, to augment therapeutic activity. Some protocols include additional chemotherapy drugs (such as vincristine and corticosteroids) during MT. The objective of ALL-MT is to ensure uninterrupted treatment at the highest tolerated doses of 6MP and MTX. This requires periodic adjustments of 6MP and MTX doses throughout treatment. Tolerance is determined through regular clinical assessments and careful monitoring of blood counts. Tolerated drug doses vary widely among patients, influenced by genetic and non-genetic factors, and require individualized dosing. Suboptimal treatment intensity in ALL-MT is associated with inferior outcomes and results from failure to treat at highest tolerated drug doses and/or interruptions in treatment due to non-adherence or toxicity. Management of MT thus requires close supervision to ensure treatment adherence, periodic drug dose modifications, and treatment to tolerance, while minimizing treatment interruptions due to toxicity. The review highlights these challenges and discusses approaches and strategies for the management of MT, focusing on the Indian context.


Similar content being viewed by others
References
Asthana S, Labani A, Mehrana A, Bakhshi S. Incidence of childhood leukemia and lymphoma in India. Pediatr Haematol Oncol J. 2018;3:115–20.
Arora RS, Bagai P, Bhakta N. Estimated national and state level incidence of childhood and adolescent cancer in India. Indian Pediatr. 2021;58:417–23.
Pui CH, Yang JJ, Hunger SP, et al. Childhood acute lymphoblastic leukemia: Progress through collaboration. J Clin Oncol. 2015;33:2938–48.
Das N, Banavali S, Bakhshi S, et al. Protocol for ICiCLe-ALL-14 (InPOG-ALL-15-01): A prospective, risk stratified, randomised, multicentre, open label, controlled therapeutic trial for newly diagnosed childhood acute lymphoblastic leukaemia in India. Trials. 2022;23:102.
Teachey DT, Hunger SP, Loh ML. Optimizing therapy in the modern age: Differences in length of maintenance therapy in acute lymphoblastic leukemia. Blood. 2021;137:168–77.
Toksvang LN, Lee SHR, Yang JJ, Schmiegelow K. Maintenance therapy for acute lymphoblastic leukemia: Basic science and clinical translations. Leukemia. 2022;36:1749–58.
Schmiegelow K, Nielsen SN, Frandsen TL, Nersting J. Mercaptopurine/Methotrexate maintenance therapy of childhood acute lymphoblastic leukemia: Clinical facts and fiction. J Pediatr Hematol Oncol. 2014;36:503–17.
Schmiegelow K. Prognostic significance of methotrexate and 6-mercaptopurine dosage during maintenance chemotherapy for childhood acute lymphoblastic leukemia. Pediatr Hematol Oncol. 1991;8:301–12.
Bhatia S, Landier W, Hageman L, et al. Systemic exposure to thiopurines and risk of relapse in children with acute lymphoblastic leukemia: A Children’s Oncology Group Study. JAMA Oncol. 2015;1:287–95.
Kato M, Ishimaru S, Seki M, et al. Long-term outcome of 6-month maintenance chemotherapy for acute lymphoblastic leukemia in children. Leukemia. 2017;31:580–4.
Somazu S, Tanaka Y, Tamai M, et al. NUDT15 polymorphism and NT5C2 and PRPS1 mutations influence thiopurine sensitivity in acute lymphoblastic leukaemia cells. J Cell Mol Med. 2021;25:10521–33.
Marinković G, Kroon J, Hoogenboezem M, et al. Inhibition of GTPase Rac1 in endothelium by 6-mercaptopurine results in immunosuppression in nonimmune cells: New target for an old drug. J Immunol. 2014;192:4370–8.
Lin TL, Vala MS, Barber JP, et al. Induction of acute lymphocytic leukemia differentiation by maintenance therapy. Leukemia. 2007;21:1915–20.
Nielsen SN, Grell K, Nersting J, et al. DNA-thioguanine nucleotide concentration and relapse-free survival during maintenance therapy of childhood acute lymphoblastic leukaemia (NOPHO ALL2008): A prospective substudy of a phase 3 trial. Lancet Oncol. 2017;18:515–24.
Bohnstedt C, Levinsen M, Rosthøj S, et al. Nordic Society of Pediatric Hematology and Oncology (NOPHO). Physician compliance during maintenance therapy in children with Down syndrome and acute lymphoblastic leukemia. Leukemia. 2013;27:866–70.
Schmiegelow K, Pulczynska M. Prognostic significance of hepatotoxicity during maintenance chemotherapy for childhood acute lymphoblastic leukaemia. Br J Cancer. 1990;61:767–72.
Levinsen M, Shabaneh D, Bohnstedt C, et al. Pneumocystis jiroveci pneumonia prophylaxis during maintenance therapy influences methotrexate/6-mercaptopurine dosing but not event-free survival for childhood acute lymphoblastic leukemia. Eur J Haematol. 2012;88:78–86.
Schultz KR, Carroll A, Heerema NA, et al. Children’s Oncology Group. Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: Children’s Oncology Group study AALL0031. Leukemia. 2014;28:1467–71.
Schmiegelow K, Nersting J, Nielsen SN, et al. Maintenance therapy of childhood acute lymphoblastic leukemia revisited-Should drug doses be adjusted by white blood cell, neutrophil, or lymphocyte counts? Pediatr Blood Cancer. 2016;63:2104–11.
Decaux G, Prospert F, Horsmans Y, Desager JP. Relationship between red cell mean corpuscular volume and 6-thioguanine nucleotides in patients treated with azathioprine. J Lab Clin Med. 2000;135:256–62.
Heerasing NM, Ng JF, Dowling D. Does lymphopenia or macrocytosis reflect 6-thioguanine levels in patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine? Intern Med J. 2016;46:465–9.
Toksvang LN, De Pietri S, Nielsen SN, et al. Hepatic sinusoidal obstruction syndrome during maintenance therapy of childhood acute lymphoblastic leukemia is associated with continuous asparaginase therapy and mercaptopurine metabolites. Pediatr Blood Cancer. 2017;64. https://doi.org/10.1002/pbc.26519.
Welch JC, Lilleyman JS. 6-Mercaptopurine dose escalation and its effect on drug tolerance in childhood lymphoblastic leukaemia. Cancer Chemother Pharmacol. 1996;38:113–6.
Landier W, Chen Y, Hageman L, et al. Comparison of self-report and electronic monitoring of 6MP intake in childhood ALL: A Children’s Oncology Group study. Blood. 2017;129:1919–26.
O’Connor D, Bate J, Wade R, et al. Infection-related mortality in children with acute lymphoblastic leukemia: An analysis of infectious deaths on UKALL2003. Blood. 2014;124:1056–61.
Kamojjala R, Bostrom B. Allopurinol to prevent mercaptopurine adverse effects in children and young adults with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2021;43:95–100.
Greenzang KA, Scavotto ML, Revette AC, Schlegel SF, Silverman LB, Mack JW. There’s no playbook for when your kid has cancer: Desired elements of an electronic resource to support pediatric cancer communication. Pediatr Blood Cancer. 2023;70:e30198.
Bansal M, Sharma KK, Vatsa M, Bakhshi S. Comparison of health-related quality of life of children during maintenance therapy with acute lymphoblastic leukemia versus siblings and healthy children in India. Leuk Lymphoma. 2013;54:1036–41.
Hill R, Hamby T, Bashore L, et al. Early nutrition intervention attenuates weight gain for pediatric acute lymphoblastic leukemia patients in maintenance therapy. J Pediatr Hematol Oncol. 2018;40:104–10.
Wadhwa A, Chen Y, Hageman L, et al. Body mass index during maintenance therapy and relapse risk in children with acute lymphoblastic leukemia: A Children’s Oncology Group report. Cancer. 2023;129:151–60.
Relling MV, Schwab M, Whirl-Carrillo M, et al. Clinical pharmacogenetics implementation consortium guideline for thiopurine dosing based on TPMT and NUDT15 genotypes: 2018 update. Clin Pharmacol Ther. 2019;105:1095–105.
Khera S, Trehan A, Bhatia P, Singh M, Bansal D, Varma M. Prevalence of TPMT, ITPA and NUDT 15 genetic polymorphisms and their relation to 6MP toxicity in north Indian children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2019;83:341–8.
Kodidela S, Dorababu P, Thakkar DN, et al. Association of NUDT15*3 and FPGS 2572C>T variants with the risk of early hematologic toxicity during 6-MP and low-dose methotrexate-based maintenance therapy in Indian patients with acute lymphoblastic leukemia. Genes (Basel). 2020;11:594.
Ramalingam R, Kaur H, Scott JX, et al. Pharmacogenetic evaluation of 6-mercaptopurine-mediated toxicity in pediatric acute lymphoblastic leukemia patients from a South Indian population. Pharmacogenomics. 2021;22:401–11.
Pai AA, Mohan A, Benjamin ESB, et al. NUDT15 c.415C>T polymorphism predicts 6-MP induced early myelotoxicity in patients with acute lymphoblastic leukemia undergoing maintenance therapy. Pharmacogenom Pers Med. 2021;14:1303–13.
Karol SE, Pei D, Smith CA, et al. Comprehensive analysis of dose intensity of acute lymphoblastic leukemia chemotherapy. Haematologica. 2022;107:371–80.
Roy Moulik N, Kumar A, Agrawal S, Mahdi AA. Folate deficiency in north Indian children undergoing maintenance chemotherapy for acute lymphoblastic leukemia-Implications and outcome. Pediatr Blood Cancer. 2018;65. https://doi.org/10.1002/pbc.26730.
Malczewska M, Kośmider K, Bednarz K, Ostapińska K, Lejman M, Zawitkowska J. Recent advances in treatment options for childhood acute lymphoblastic leukemia. Cancers (Basel). 2022;14:2021.
Ganguly S, Bakhshi S. Teleconsultations and shared care in pediatric oncology during COVID-19. Indian J Pediatr. 2021;88:1–2.
Mungle T, Gogoi MP, Mitra S, et al. Developing an automated dose advice programme to assist adaptive antimetabolite dose decisions during maintenance therapy in acute lymphoblastic leukaemia. Pediatr Hematol Oncol J. 2020;5:S10.
Larsen RH, Hjalgrim LL, Grell K, et al. Pharmacokinetics of tablet and liquid formulations of oral 6-mercaptopurine in children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2020;86:25–32.
Schmiegelow K, Ifversen M. Myelotoxicity, pharmacokinetics, and relapse rate with methotrexate/6-mercaptopurine maintenance therapy of childhood acute lymphoblastic leukemia. Pediatr Hematol Oncol. 1996;13:433–41.
Kamle A, Ghara N, Ghosh D, Gogoi MP, Jana B, Mungle T. Allopurinol adjuvant in acute lymphoblastic leukaemia maintenance treatment. Pediatr Hematol Oncol J. 2022;7:S48.
Larsen RH, Utke Rank C, Grell K, et al. Increments in DNA-thioguanine level during thiopurine-enhanced maintenance therapy of acute lymphoblastic leukemia. Haematologica. 2021;106:2824–33.
Arora RS, Bakhshi S. Indian Pediatric Oncology Group (InPOG) – Collaborative research in India comes of age. Pediatr Hematol Oncol J. 2016;1:13–7.
Acknowledgements
The authors gratefully acknowledge the insights gained from patients and families in the course of supervising their clinical care, from colleagues in the clinical (Dr. Niharendu Ghara, Dr. Debjani Ghosh; Pediatric Hematology and Oncology) and research units (Dr. Jasmeet Sidhu, Mr. Parag Das, Mr. Bishwaranjan Jana, Ms. Prakriti Roy; Tata Translational Cancer Research Centre) at the Tata Medical Center, Kolkata, and from research collaborations with the Indian Institute of Technology, Kharagpur (Prof. Sangeeta Das Bhattacharya, Prof. Jayanta Mukhopadhyay), the Indian Statistical Institute, Kolkata (Prof. Kiranmoy Das), the Postgraduate Institute of Medical Education & Research, Chandigarh (Prof. Amita Trehan, Dr. Prateek Bhatia) and the Tata Consultancy Services (Ms. Anju Goel, Mr. Amit Saxena).
Funding
This work was supported in part by grants from the National Cancer Grid (2016/001) and the Indian Council of Medical Research (79/159/2015/NCD-III) for the Indian Collaborative Childhood Leukaemia ICiCLe-ALL-14 collaborative multicentre paediatric ALL clinical trial, funding from the UKIERI / SPARC programme (UK-India Education and Research Initiative / Scheme for Promotion of Academic and Research Collaboration; IIT/SRIC/MM/IOS_SKI/2018-19/325) awarded jointly to IIT Kharagpur (Prof SD Bhattacharya) and the University of Manchester (Prof. V Saha) and a Wellcome-Department of Biotechnology India Alliance Fellowship to Prof. Vaskar Saha (IA/M/12/500261).
Author information
Authors and Affiliations
Contributions
SK wrote the paper; AM and TM prepared the Figures; MPG prepared the Tables; SK and VS reviewed the final draft of the paper. All authors have reviewed and approved the final version of the manuscript submitted for publication. VS will act as guarantor for this manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Krishnan, S., Mahadevan, A., Mungle, T. et al. Maintenance Treatment in Acute Lymphoblastic Leukemia: A Clinical Primer. Indian J Pediatr 91, 47–58 (2024). https://doi.org/10.1007/s12098-023-04687-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-023-04687-6