Abstract
Objectives
There is sufficient evidence to support use of caffeine therapy for apnea of prematurity, but practices vary widely when it comes to discontinuing therapy. This study was planned to compare ‘recurrence of apnea of prematurity’ (RAP); when 2 protocols were used to stop caffeine therapy.
Methods
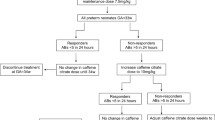
Neonates delivered at 26–32 wk gestation on caffeine therapy for apnea of prematurity were randomized into 2 groups: Group 1—caffeine stopped at 7 d apnea-free period, and Group 2—continued for a prefixed period till at least 34 wk postmenstrual age (PMA). Proportion of infants in each group with RAP were analyzed.
Results
Each group consisted of 60 infants. Proportion of infants in each group with RAP, were not different (15% vs 13%); odds ratio (OR) 0.87; 95% confidence interval (CI) (0.31–2.43). Caffeine could be stopped earlier (33 vs 34 wk PMA); and cumulative duration of therapy was lesser (19.5 vs 33 d) when stopped at 7 d apnea-free period. Other studied outcomes were similar between the two groups.
Conclusions
Mandatorily continuing caffeine therapy up to 34 wk PMA in select preterm groups does not seem to decrease risk of recurrence of apnea. Larger trials that specifically study extremely preterm infants are required to make robust recommendations on when to stop therapy.
Clinical Trials Registry of India no
CTRI/2016/12/007559. http://ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=14195&EncHid=&modid=&compid=%27,%2714195det%27

Similar content being viewed by others
References
Schmidt B, Roberts RS, Davis P, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354(20):2112–21.
Hsieh EM, Hornik CP, Clark RH, Laughon MM, Benjamin DK, Smith PB; On behalf of the Best Pharmaceuticals for Children Act- Pediatrics trials network. Medication use in the neonatal intensive care unit. Am J Perinatol. 2014;31(9):811–22.
Schmidt B, Roberts RS, Davis P, et al. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357(19):1893–902.
Lodha A, Seshia M, McMillan DD, et al. Association of early caffeine administration and neonatal outcomes in very preterm neonates. JAMA Pediatr. 2015;169:33–8.
Kattwinkel J, Nattie C, Robinson M. Margin of safety for discharge after apnea in preterm infants. Pediatrics. 1997;100:795–801.
Picone S, Bedetta M, Paolillo P. Caffeine citrate: when and for how long. A literature review. J Matern Fetal Neonatal Med. 2012;25:11–4.
Al Ansari E, Qeretli R, Fayed M, Altammami H. Caffeine therapy practice in the management of apnea of prematurity: national survey in Saudi Arabia. J Clin Neonatol. 2018;7:217–23.
Natarajan G, Botica ML, Thomas R, Aranda JV. Therapeutic drug monitoring for caffeine in preterm neonates: an unnecessary exerceise? Pediatrics. 2007;119:936–40.
Atik A, Harding R, De Matteo R, et al. Caffeine for apnea of prematurity: effects on the developing brain. Neurotoxicol. 2016;58:94–102.
Bauer J, Maier K, Linderkamp O, Hentschel R. Effect of caffeine on oxygen consumption and metabolic rate in very low birth weight infants with idiopathic apnea. Pediatrics. 2001;107(4):660–3.
Rhein LM, Dobson NR, Darnall RA, et al. Effects of caffeine on intermittent hypoxia in infants born prematurely: a randomized controlled trial. JAMA Pediatr. 2014;168:250–7.
Lin Z, Green RS, Chen S, et al. Quantification of EUGR as a measure of the quality of nutritional care of premature infants. PLoS One. 2015;10(7):e0132584.
Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin N Am. 1986;33(1):179–201.
Ehrenkranz RA, Walsh MC, Vohr BR, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116(6):1353–60.
Early treatment for Retinopathy of Prematurity Cooperative Group. Revised indication for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–94.
Lorch SA, Srinivasan L, Escobar GJ. Epidemiology of apnea and bradycardia resolution in premature infants. Pediatrics. 2011;128(2):e366–73.
Prakash R, Pournami F, Prabhakar J, et al. G573(P) ‘How long is not short?’: Duration of caffeine therapy in preterm infants- a randomized controlled trial. Arch Dis Child. 2020;105:A205–6.
Aranda JV, Gorman W, Bergsteinsson H, Gunn T. Efficacy of caffeine in treatment of apnea in the low-birth-weight infant. J Pediatr. 1977;90(3):467–72.
Abdel-Hady A, Nasef N, Shabaan AE, Nour I. Caffeine therapy in preterm infants. World J Clin Pediatr. 2015;4(4):81–93.
Eichenwald EC, Committee on fetus and newborn, American Academy of Pediatrics. Apnea of prematurity. Pediatrics. 2016;137(1):e20153757.
Eichenwald EC, Aina A, Stark AR. Apnea frequently persists beyond term gestation in infants delivered at 24 to 28 weeks. Pediatrics. 1997;100(3):354–9.
Martin RJ, Wang K, Köroğlu O, Di Fiore J, Kc P. Intermittent hypoxic episodes in preterm infants: do they matter? Neonatology. 2011;100(3):303–10.
Poets CF, Roberts RS, Schmidt B, et al. Association between intermittent hypoxemia or bradycardia and late death or disability in extremely preterm infants. JAMA. 2015;314(6):595–603.
Butler TJ, Firestone KS, Grow JL, Kantak AD. Standardizing documentation and the clinical approach to apnea of prematurity reduces length of stay, improves staff satisfaction, and decreases hospital cost. Jt Comm J Qual Patient Saf. 2014;40(6):263–9.
Author information
Authors and Affiliations
Contributions
NJ conceived the study; NJ, JP, PMCN planned and designed the study protocol; RP (principle investigator) collected data; RP, NJ, AN and FP contributed to statistical analysis and interpretation; RP, FP drafted the manuscript. All authors approved the final manuscript. NJ will act as guarantor for this paper.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 68 kb)
Rights and permissions
About this article
Cite this article
Prakash, R., Pournami, F., Prabhakar, J. et al. Duration of Caffeine for Apnea of Prematurity—A Randomized Controlled Trial. Indian J Pediatr 88, 1174–1179 (2021). https://doi.org/10.1007/s12098-021-03659-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-021-03659-y