Abstract
Objectives
To investigate the effectiveness of low dose secondary/tertiary prophylaxis in severe Hemophilia A children and determine improvements in their daily life.
Methods
Thirty Hemophilia A children (≤ 12 y) with factor VIII <2% and less than two joint bleeds without inhibitors, were given prophylaxis with recombinant Fc fusion long acting factor VIII (ELOCTATE) at 10 IU.kg−1 twice weekly for 1 y. Earlier, patients received on-demand FVIII for a minimum of six months. Outcome was measured in terms of annual bleeding rate, Hemophilia Joint Health Score (HJHS) and child activity/participation was measured in terms of school absenteeism, School Activity Participation Score and Daily Activity Score according to Beijing Children Hospital assessment scale.
Results
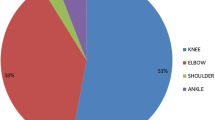
A total of 30 children were included in the study. There was reduction in annual joint bleeds by 85.76% (14.5 to 2.2) and school absenteeism (days/month) by 86% (17.38 to 2.42) before and after prophylaxis respectively. Majority (43%) showed moderate improvement in daily activity score. Mean HJHS score was 8.3. There was mild improvement in School Activity Participation Score in 57%. Mean annual hospitalization rate reduced from 8.7 to 1.1 with improvement in joint scores. Mean annual factor consumption decreased from 1944.2 IU.kg−1 to 1560.3 IU.kg−1.
Conclusions
With low dose secondary/tertiary prophylaxis, there is significant reduction in the annual joint bleed rate with improvement in joint health and child activity. As factor consumption is reduced, this has a positive effect on cost benefit; and is a very feasible option in developing countries.




Similar content being viewed by others
References
Haldane JBS. The rate of spontaneous mutation of a human gene. J Genet. 1935;31:317.
Kar A, Phadnis S, Dharmarajan S, Nakade J. Epidemiology & social costs of haemophilia in India. Indian J Med Res. 2014;140:19–31.
Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al; Treatment Guidelines Working Group on Behalf of the World Federation of Hemophilia. Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1–47.
Hilliard P, Zourikian N, Blanchette V, et al. Musculoskeletal health of subjects with hemophilia a treated with tailored prophylaxis: Canadian Hemophilia primary Prophylaxis (CHPS) Study. J Thromb Haemost. 2013;11:460–6.
Astermark J, Petrini P, Tengborn L, Schulman S, Ljung R, Berntorp E. Primary prophylaxis in severe haemophilia should be started at an early age but can be individualized. Br J Haematol. 1999;105:1109–13.
Nilsson IM, Berntorp E, Lofqvist T, Pettersson H. Twenty-five years’ experience of prophylactic treatment in severe haemophilia A and B. J Intern Med. 1992;232:25–3.
Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535–44.
Van Creveld S. Prophylaxis of joint hemorrhages in hemophilia. Acta Haematol. 1971;45:120–7.
Wu R, Luke KH, Poon MC, et al. Low dose secondary prophylaxis reduces joint bleeding in severe and moderate haemophilic children: a pilot study in China. Haemophilia. 2011;17:70–4.
Tang L, Wu R, Sun J, et al. Short-term low-dose secondary prophylaxis for severe/moderate haemophilia a children is beneficial to reduce bleed and improve daily activity, but there are obstacles in its execution: a multi-centre pilot study in China. Haemophilia. 2013;19:27–34.
Gouider E, Jouini L, Achour M, et al. Low dose prophylaxis in Tunisian children with haemophilia. Haemophilia. 2017;23:77–81.
Verma S, Dutta T, Subramanian N, et al. A randomised study of very low dose FVIII prophylaxis in severe haemophilia- a success story from a resource limited country. Haemophilia. 2016;22:342–8.
Sidharthan N, Pillai VN, Mathew S, et al. Low dose secondary/tertiary prophylaxis is feasible and effective in resource limited setting in South India for children with hemophilia. Blood. 2016;128:2336.
Oldenburg J. Optimal treatment strategies for hemophilia: achievements and limitations of current prophylactic regimens. Blood. 2015;125:2038–44.
Singh T, Sharma S, Nagesh S. Socio-economic status scales updated for 2017. Int J Res Med Sci. 2017;5:3264–7.
Eshghi P, Sadeghi E, Tara SZ, Habibpanah B, Hantooshzadeh R. Iranian low-dose escalating prophylaxis regimen in children with severe hemophilia A and B. Clin Appl Thromb Hemost. 2018;24:513–8.
Mahlangu J, Powell JS, Ragni MV, et al; A-LONG Investigators. Phase 3 study of rFVIIIFc in severe hemophilia A. Blood. 2014;123:317–25.
Sommer JM, Moore N, McGuffie-Valentine B, et al. Comparative field study evaluating the activity of recombinant factor VIII Fc fusion protein in plasma samples at clinical haemostasis laboratories. Haemophilia. 2014;20:294–300.
Fischer K. Low-dose prophylaxis for severe haemophilia: a little goes a long way. Haemophilia. 2016;22:331–3.
Young NL, Wakefield C, Burke TA, Ray R, McCusker PJ, Blanchette V. Updating the Canadian hemophilia outcomes-kids life assessment tool (CHO-KLAT Version2.0). Value Health. 2013;16:837–41.
Author information
Authors and Affiliations
Contributions
SG, PKM, PC: Concept, design, literature search, clinical studies, acquisition, analysis or interpretation of data, statistical analysis, manuscript preparation, manuscript editing and review; AP, SB, RD, TKD: Literature search, clinical studies, manuscript editing and review. PC will act as guarantor for this paper.
Corresponding author
Ethics declarations
Informed Consent
Informed consent was obtained from all individual participants and/or their legal guardians included in the study.
Conflict of Interest
None.
Source of Funding
The study was conducted with long acting recombinant FVIII Fc fusion (ELOCTATE, Biogen Inc., Cambridge, MA 02142) products from World Federation of Hemophilia (WFH) Humanitarian Aid.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gulshan, S., Mandal, P.K., Phukan, A. et al. Is Low Dose a New Dose to Initiate Hemophilia A Prophylaxis? – A Systematic Study in Eastern India. Indian J Pediatr 87, 345–352 (2020). https://doi.org/10.1007/s12098-019-03179-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-019-03179-w