Abstract
Objective
To investigate the dynamic changes of platelet-derived microparticles (PDMP) in Kawasaki disease (KD) and its clinical significance and to study its relationship with intravenous immunoglobulin (IVIG) resistance, inflammatory indicators and aspirin treatment in children with KD.
Methods
Twenty children with KD were enrolled as the experimental group, while 20 age- and gender-matched children with common febrile disease were included in the control group. Blood samples were drawn before and 7–10 d after IVIG infusion and thereafter at 1, 2, and 3 mo after the onset of KD to estimate the PDMP concentrations by enzyme linked immunosorbent assay (ELISA). C-reactive protein (hs-CRP), erythrocyte sedimentation rate (ESR), procalcitonin (PCT), and cytokines [Interleukin-6 (IL-6), Tumor necrosis factor-α (TNF-α), and Soluble interleukin-2 (sIL-2R)] were also measured.
Results
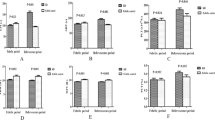
The level of PDMP in KD children before IVIG was significantly higher than that in controls (P < 0.0001). The PDMP level in KD children decreased significantly at 7 to 10 d after IVIG (P < 0.0001) and then decreased to the lowest level in the course of 1 to 2 mo. Some children’s PDMP level rebounded in the course of 3 mo (P = 0.047). In addition, the mean level of PDMP in IVIG-resistant children was higher than that in IVIG-effective children; however, there was no significant difference between the two groups (P = 0.1945). Furthermore, PDMP was positively correlated with hs-CRP, IL-6, and sIL-2R levels, but no correlation was observed with ESR, PCT, and TNF-α levels.
Conclusions
PDMP can be used as an index to monitor inflammation in children at the acute stage of KD. And the duration of platelet activation in KD is individualized.



Similar content being viewed by others
References
Rowley AH, Shulman ST. Pathogenesis and management of Kawasaki disease. Expert Rev Anti-Infect Ther. 2010;8:197–203.
Takahashi K, Oharaseki T, Yokouchi Y. Pathogenesis of Kawasaki disease. Clin Exp Immunol. 2011;164:20–2.
Lin YJ, Chang JS, Liu X, et al. Genetic variants in PLCB4/PLCB1 as susceptibility loci for coronary artery aneurysm formation in Kawasaki disease in Han Chinese in Taiwan. Sci Rep. 2015;5:14762.
Li JS, Newburger JW. Antiplatelet therapy in pediatric cardiovascular patients. Pediatr Cardiol. 2010;31:454–61.
Tamir A, Sorrentino S, Motahedeh S, et al. The macromolecular architecture of platelet-derived microparticles. J Struct Biol. 2016;193:181–7.
Italiano JJ, Mairuhu AT, Flaumenhaft R. Clinical relevance of microparticles from platelets and megakaryocytes. Curr Opin Hematol. 2010;17:578–84.
Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 2007;21:157–71.
Kim HK, Song KS, Chung JH, Lee KR, Lee SN. Platelet microparticles induce angiogenesis in vitro. Br J Haematol. 2004;124:376–84.
Boulanger CM, Amabile N, Tedgui A. Circulating microparticles: a potential prognostic marker for atherosclerotic vascular disease. Hypertension. 2006;48:180–6.
Preston RA, Jy W, Jimenez JJ, et al. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension. 2003;41:211–7.
van der Zee PM, Biro E, Ko Y, et al. P-selectin- and CD63-exposing platelet microparticles reflect platelet activation in peripheral arterial disease and myocardial infarction. Clin Chem. 2006;52:657–64.
Diehl P, Nagy F, Sossong V, et al. Increased levels of circulating microparticles in patients with severe aortic valve stenosis. Thromb Haemost. 2008;99:711–9.
Burbano C, Rojas M, Vasquez G, et al. Microparticles that form immune complexes as modulatory structures in autoimmune responses. Mediat Inflamm. 2015;2015:267590.
Singh S, Vignesh P, Burgner D. The epidemiology of Kawasaki disease: a global update. Arch Dis Child. 2015;100:1084–8.
McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–99.
Li ZY, Lou JG, Chen J. Analysis of primary symptoms and disease spectrum in Epstein-Barr virus infected children. Zhonghua Er Ke Za Zhi. 2004;42:20–2.
Chandrashekar L, Rajappa M, Revathy G, et al. Is enhanced platelet activation the missing link leading to increased cardiovascular risk in psoriasis? Clin Chim Acta. 2015;446:181–5.
Hsu J, Gu Y, Tan SL, Narula S, DeMartino JA, Liao C. Bruton's tyrosine kinase mediates platelet receptor-induced generation of microparticles: a potential mechanism for amplification of inflammatory responses in rheumatoid arthritis synovial joints. Immunol Lett. 2013;150:97–104.
Hayon Y, Dashevsky O, Shai E, Brill A, Varon D, R. Leker R. Platelet microparticles induce angiogenesis and neurogenesis after cerebral ischemia. Curr Neurovasc Res. 2012;9:185–92.
Denzer K, Kleijmeer MJ, Heijnen HF, et al. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113:3365–74.
Flaumenhaft R, Dilks JR, Richardson J, et al. Megakaryocyte-derived microparticles: direct visualization and distinction from platelet-derived microparticles. Blood. 2009;113:1112–21.
Smalley DM, Root KE, Cho H, Ross MM, Ley K. Proteomic discovery of 21 proteins expressed in human plasma-derived but not platelet-derived microparticles. Thromb Haemost. 2007;97:67–80.
Dean WL, Lee MJ, Cummins TD, Schultz DJ, Powell DW. Proteomic and functional characterisation of platelet microparticle size classes. Thromb Haemost. 2009;102:711–8.
Sinauridze EI, Kireev DA, Popenko NY, et al. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97:425–34.
Burger D, Schock S, Thompson CS, Montezano AC, Hakim AM, Touyz RM. Microparticles: biomarkers and beyond. Clin Sci (Lond). 2013;124:423–41.
Drutskaya MS, Efimov GA, Kruglov AA, Nedospasov SA. Can we design a better anti-cytokine therapy? J Leukoc Biol. 2017;102:783–90.
Larsen SB, Grove EL, Wurtz M, et al. The influence of low-grade inflammation on platelets in patients with stable coronary artery disease. Thromb Haemost. 2015;114:519–29.
Kim HJ, Choi EH, Lim YJ, Kil HR. The usefulness of platelet-derived microparticle as biomarker of antiplatelet therapy in Kawasaki disease. J Korean Med Sci. 2017;32:1147–53.
Kimura M, Harazaki M, Fukuoka T, et al. Targeted use of prednisolone with the second IVIG dose for refractory Kawasaki disease. Pediatr Int. 2017;59:397–403.
Lee HY, Song MS. Predictive factors of resistance to intravenous immunoglobulin and coronary artery lesions in Kawasaki disease. Korean J Pediatr. 2016;59:477–82.
Seki M, Kobayashi T, Kobayashi T, et al. External validation of a risk score to predict intravenous immunoglobulin resistance in patients with Kawasaki disease. Pediatr Infect Dis J. 2011;30:145–7.
Kobayashi T, Inoue Y, Takeuchi K, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113:2606–12.
Yahata T, Suzuki C, Yoshioka A, Hamaoka A, Ikeda K. Platelet activation dynamics evaluated using platelet-derived microparticles in Kawasaki disease. Circ J. 2014;78:188–93.
Acknowledgments
The authors are grateful to the participants and their parents for their commitment to this project. This study utilized clinical research facilities and resources provided by the Institute of Pediatrics in Children’s Hospital of Nanjing Medical University.
Contributions
HY: Principal investigator of the project. Responsible for planning out the trial, obtaining funds, and overall conduct of the trial. He takes full responsibility for the data and their accuracy and the conduct of the research. JJ: She is responsible for performing the experiment and major proportion of writing the article. JW: Responsible for the generation of data analysis and writing of results in this article. YL: He is also responsible for performing the experiment and major proportion of writing the article. NH: She is responsible for selection of subjects and collection of blood samples. ZF: Responsible for treatment of samples. LM: Responsible for communication with parents and application for Institutional Ethics Committee. HY will act as guarantor for this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest
None.
Rights and permissions
About this article
Cite this article
Jin, J., Wang, J., Lu, Y. et al. Platelet-Derived Microparticles: A New Index of Monitoring Platelet Activation and Inflammation in Kawasaki Disease. Indian J Pediatr 86, 250–255 (2019). https://doi.org/10.1007/s12098-018-2765-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-018-2765-2