Abstract
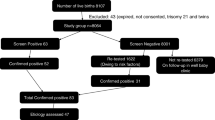
The Indian Society for Pediatric and Adolescent Endocrinology has formulated Clinical Practice Guidelines for newborn screening, diagnosis and management of congenital hypothyroidism (CH). This manuscript, part II addresses management and follow-up. Recommendations: Screening should be done for every newborn using cord blood, or postnatal blood ideally at 48 to 72 h of age. Neonates with screen TSH > 20 mIU/L serum units (or >34 mIU/L for samples taken between 24 and 48 h of age) should be recalled for confirmation. For screen TSH > 40 mIU/L, immediate confirmatory venous T4/FT4 and TSH, and for mildly elevated screen TSH, a second screening TSH at 7 to 10 d of age, should be taken. Preterm and low birth weight infants should undergo screening at 48–72 h age. Sick babies should be screened at least by 7 d of age. Venous confirmatory TSH >20 mIU/L before age 2 wk and >10 mIU/L after age 2 wk, with low T4 (<10 μg/dL) or FT4 (<1.17 ng/dL) indicate primary CH and treatment initiation. Imaging is recommended by radionuclide scintigraphy and ultrasonography after CH is biochemically confirmed but treatment should not be delayed till scans are performed. Levothyroxine is commenced at 10-15 μg/kg in the neonatal period. Serum T4/FT4 is measured at 2 wk and TSH and T4/FT4 at 1 mo, then 2 monthly till 6 mo, 3 monthly from 6 mo–3 y and every 3–6 mo thereafter. Babies with the possibility of transient CH should be re-evaluated at age 3 y, to assess the need for lifelong therapy.
Similar content being viewed by others
Abbreviations
- CH:
-
Congenital hypothyroidism
- NBS:
-
Newborn screening
- LT4:
-
Levothyroxine
- DBS:
-
Dried blood spot
- ISPAE:
-
Indian Society for Pediatric and Adolescent Endocrinology
- TSH:
-
Thyroid stimulating hormone
- T4:
-
Thyroxine
- FT4:
-
Free thyroxine
References
Mathai S. Newborn screening for congenital hypothyroidism- experience from India. Abstract presented at 8th Asia Pacific Regional Meeting of the International Society for Neonatal Screening. New Delhi, Sep 2013.
Rama Devi AR. Newborn screening in India, experience from pilot initiative (ICMR Multicenter Project). Abstract presented at 8th Asia Pacific Regional Meeting of the International Society for Neonatal Screening. New Delhi, Sep 2013.
Agarwal M, Joshi K, Bhatia V, et al. Feasibility study of an outreach program of newborn screening program in Uttar Pradesh. Indian J Pediatr. 2015;82:427–32.
Rose SR, Brown RS, Foley T, et al. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics. 2006;117:2290–303.
Léger J, Olivieri A, Donaldson M, et al; ESPE-PES-SLEP-JSPE-APEG-APPES-ISPAE; Congenital Hypothyroidism Consensus Conference Group. European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. J Clin Endocrinol Metab. 2014;99:363–84.
Hindmarsh PC. Optimisation of thyroxine dose in congenital hypothyroidism. Arch Dis Child. 2002;86:73–5.
Bongers-Schokking JJ, de Muinck Keizer-Schrama SM. Influence of timing and dose of thyroid hormone replacement on mental, psychomotor, and behavioral development in children with congenital hypothyroidism. J Pediatr. 2005;147:768–74.
Bongers-Schokking JJ, Resing WC, de Rijke YB, de Ridder MA. de Muinck Keizer-Schrama SM. Cognitive development in congenital hypothyroidism: is overtreatment a greater threat than undertreatment? J Clin Endocrinol Metab. 2013;98:4499–506.
Swiglo BA, Murad MH, Schunemann HJ, et al. A case for clarity, consistency and helpfulness: state-of-the-art clinical practice guidelines in endocrinology using the grading of recommendations, assessment, development, and evaluation system. J Clin Endocrinol Metab. 2008;93:666–73.
Chang Y, Lee DH, Hong YH, Hong HS, Choi DS, Seo DY. Congenital hypothyroidism: analysis of discordant US and scintigraphic findings. Radiology. 2011;258:872–9.
Salerno M, Militerni R, Bravaccio C, et al. Effect of different starting doses of levothyroxine on growth and intellectual outcome at four years of age in congenital hypothyroidism. Thyroid. 2002;12:45–52.
Schoen EJ, Clapp W. To TT, Fireman BH. The key role of newborn thyroid scintigraphy with isotopic Iodide (123I) in defining and managing congenital hypothyroidism. Pediatrics. 2004;114:e683–8.
Rovert J, Ehrlich R, Sorbara D. Intellectual outcome in children with fetal hypothyroidism. J Pediatr. 1987;110:700–4.
Kempers MJ, van der Sluijs Veer L, Nijhuis-van der Sanden MW, et al. Intellectual and motor development of young adults with congenital hypothyroidism diagnosed by neonatal screening. J Clin Endocrinol Metab. 2006;91:418–24.
Irving SA, Vadiveloo T, Leese GP. Drugs that interact with levothyroxine: an observational study from the thyroid epidemiology, audit and research study (TEARS). Clin Endocrinol. 2015;82:136–41.
Olivieri A, Stazi MA, Mastroiacovo P, et al. A population-based study on the frequency of additional congenital malformations in infants with congenital hypothyroidism: data from the Italian Registry for congenital hypothyroidism (1991–1998). J Clin Endocrinol Metab. 2002;87:557–62.
Contributions
MD, RS, SS, RP, RI, VB reviewed the literature, drafted the manuscript, obtained extensive inputs from the editorial team, and finalized the manuscript incorporating these inputs. VB will act as the guarantor of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Source of Funding
None.
Additional information
Writing Committee: S Sudhanshu, I Riaz, R Sharma, MP Desai, R Parikh, V Bhatia
Editorial committee: G Gupta, G Jevalikar, S Mathai, PSN Menon, P Raghupathy, S Rao, A Seth, A Simon, M Vijayakumar, A Virmani
Electronic supplementary material
ESM 1
(PDF 173 kb)
Rights and permissions
About this article
Cite this article
Sudhanshu, S., Riaz, I., Sharma, R. et al. Newborn Screening Guidelines for Congenital Hypothyroidism in India: Recommendations of the Indian Society for Pediatric and Adolescent Endocrinology (ISPAE) – Part II: Imaging, Treatment and Follow-up. Indian J Pediatr 85, 448–453 (2018). https://doi.org/10.1007/s12098-017-2576-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-017-2576-x