Abstract
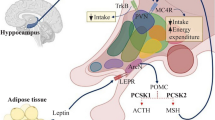
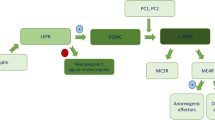
The aim of this article is to provide an in depth review of the rare genetic and syndromic forms of childhood obesity. The authors demonstrate the complexity and inter-relationships of the leptin-melanocortin signaling pathway and its central nervous system and systemic effects. Authors highlight the clinical distinctive features of genetic/syndromic causes for childhood obesity, in particular, relative shorter height to their genetic potential, developmental challenges and in some instances, ophthalmological and retina changes. They outline specific genetic testing and treatment options available for these conditions.

Similar content being viewed by others
References
Farooqi S. Insights from the genetics of severe childhood obesity. Horm Res. 2007;68:5–7. ed. 2007;356:237–47.
Farooqi IS, Wangensteen T, Collins S, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. 2007;356:237–47.
Wabitsch M, Funcke JB, Lennerz B, et al. Biologically inactive leptin and early-onset extreme obesity. N Engl J Med. 2015;372:48–54.
Wabitsch M, Funcke JB, von Schnurbein J, et al. Severe early-onset obesity due to bioinactive leptin caused by a p.N103k mutation in the leptin gene. J Clin Endocrinol Metab. 2015;100:3227–30.
Abaci A, Catli G, Bayram E, et al. A case of rapid-onset obesity with hypothalamic dysfunction, hypoventilation, autonomic dysregulation, and neural crest tumor: Rohhadnet syndrome. Endocr Pract. 2013;19:e12–6.
Jacobson P, Ukkola O, Rankinen T, et al. Melanocortin 4 receptor sequence variations are seldom a cause of human obesity: the swedish obese subjects, the heritage family study, and a memphis cohort. J Clin Endocrinol Metab. 2002;87:4442–6.
Vollbach H, Brandt S, Lahr G, et al. Prevalence and phenotypic characterization of MC4R variants in a large pediatric cohort. Int J Obes. 2017;41:13–22.
Hinney A, Volckmar AL, Knoll N. Melanocortin-4 receptor in energy homeostasis and obesity pathogenesis. Prog Mol Biol Transl Sci. 2013;114:147–91.
Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–95.
Paolini B, Maltese PE, Del Ciondolo I, et al. Prevalence of mutations in LEP, LEPR, and MC4R genes in individuals with severe obesity. Genet Mol Res. 2016;15(3). https://doi.org/10.4238/gmr.15038718.
Hainerova IA, Lebl J. Treatment options for children with monogenic forms of obesity. World Rev Nutr Diet. 2013;106:105–12.
Wardlaw SL. Hypothalamic proopiomelanocortin processing and the regulation of energy balance. Eur J Pharmacol. 2011;660:213–9.
Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by pomc mutations in humans. Nat Genet. 1998;19:155–7.
Kuehnen P, Mischke M, Wiegand S, et al. An alu element-associated hypermethylation variant of the pomc gene is associated with childhood obesity. PLoS Genet. 2012;8:e1002543.
Kuhnen P, Handke D, Waterland RA, et al. Interindividual variation in DNA methylation at a putative pomc metastable epiallele is associated with obesity. Cell Metab. 2016;24:502–9.
Kuhnen P, Clement K, Wiegand S, et al. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N Engl J Med. 2016;375:240–6.
Loffler D, Behrendt S, Creemers JW, et al. Functional and clinical relevance of novel and known pcsk1 variants for childhood obesity and glucose metabolism. Mol Metab. 2017;6:295–305.
Jackson RS, Creemers JW, Ohagi S, et al. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet. 1997;16:303–6.
Khan MJ, Gerasimidis K, Edwards CA, Shaikh MG. Mechanisms of obesity in Prader-Willi syndrome. Pediatr Obes. 2016; https://doi.org/10.1111/ijpo.12177.
Holm VA, Cassidy SB, Butler MG, et al. Prader-Willi syndrome: consensus diagnostic criteria. Pediatrics. 1993;91:398–402.
Driscoll DJ, Miller JL, Schwartz S, Cassidy SB. Prader-Willi syndrome. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean, LJH, Bird TD, Ledbetter N, Mefford HC, Smith RJH, Stephens K, editors. GeneReviews® [Internet]. Seattle: University of Washington, Seattle; 1993-2017.
Ize-Ludlow D, Gray JA, Sperling MA, et al. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation presenting in childhood. Pediatrics. 2007;120:e179–88.
Chew HB, Ngu LH, Keng WT. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation and autonomic dysregulation (rohhad): a case with additional features and review of the literature. BMJ Case Rep. 2011;2011.
Chandrakantan A, Poulton TJ. Anesthetic considerations for rapid-onset obesity, hypoventilation, hypothalamic dysfunction, and autonomic dysfunction (rohhad) syndrome in children. Paediatr Anaesth. 2013;23:28–32.
Sumanasena SP, de Silva S, Perera I, Sudeen A, Wasala R. Rapid onset obesity, hypoventilation, hypothalamic, autonomic and thermal dysregulation, and neural tumour (rohhadnet) syndrome presenting with cushing syndrome. Ceylon Med J. 2012;57:47–8.
Barclay SF, Rand CM, Borch LA, et al. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation (rohhad): exome sequencing of trios, monozygotic twins and tumours. Orphanet J Rare Dis. 2015;10:103.
Barclay SF, Rand CM, Gray PA, et al. Absence of mutations in hcrt, hcrtr1 and hcrtr2 in patients with rohhad. Respir Physiol Neurobiol. 2016;221:59–63.
Alvarez-Satta M, Castro-Sanchez S, Valverde D. Alstrom syndrome: current perspectives. Appl Clin Genet. 2015;8:171–9.
Nikopoulos K, Butt GU, Farinelli P, et al. A large multiexonic genomic deletion within the ALMS1 gene causes Alstrom syndrome in a consanguineous Pakistani family. Clin Genet. 2015; https://doi.org/10.1111/cge.12645.
Das Bhowmik A, Gupta N, Dalal A, Kabra M. Whole exome sequencing identifies a homozygous nonsense variation in ALMS1 gene in a patient with syndromic obesity. Obes Res Clin Pract. 2017;11:241–6.
Forsythe E, Beales PL. Bardet-biedl syndrome. Eur J Hum Genet. 2013;21:8–13.
Seo S, Guo DF, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum Mol Genet. 2009;18:1323–31.
Gruters-Kieslich A, Reyes M, Sharma A, et al. Early-onset obesity: unrecognized first evidence for GNAS mutations and methylation changes. J Clin Endocrinol Metab. 2017;102:2670–7.
Mason K, Page L, Balikcioglu PG. Screening for hormonal, monogenic, and syndromic disorders in obese infants and children. Pediatr Ann. 2014;43:e218–24.
Ali O, Cerjak D, Kent JW Jr, et al. Methylation of SOCS3 is inversely associated with metabolic syndrome in an epigenome-wide association study of obesity. Epigenetics. 2016;11:699–707.
Farooqi IS, Jebb SA, Langmack G, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–84.
Styne DM, Arslanian SA, Connor EL, et al. Pediatric obesity-assessment, treatment, and prevention: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:709–57.
Yanovski JA, Krakoff J, Salaita CG, et al. Effects of metformin on body weight and body composition in obese insulin-resistant children: a randomized clinical trial. Diabetes. 2011;60:477–85.
Kendall D, Vail A, Amin R, et al. Metformin in obese children and adolescents: the moca trial. J Clin Endocrinol Metab. 2013;98:322–9.
Maahs D, de Serna DG, Kolotkin RL, et al. Randomized, double-blind, placebo-controlled trial of orlistat for weight loss in adolescents. Endocr Pract. 2006;12:18–28.
Chanoine JP, Hampl S, Jensen C, Boldrin M, Hauptman J. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA. 2005;293:2873–83.
Contributions
IHK drafted the manuscript and developed it ready for submission. IHK is the corresponding author. CR provided oversight of manuscript editing and developed the included figure and Table 1. Senior author. Dr. Catherine Pihoker, Professor of Pediatrics / University of Washington, Division of Endocrinology and Diabetes, Seattle Children’s Hospital will act as guarantor for this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Source of Funding
None.
Rights and permissions
About this article
Cite this article
Koves, I.H., Roth, C. Genetic and Syndromic Causes of Obesity and its Management. Indian J Pediatr 85, 478–485 (2018). https://doi.org/10.1007/s12098-017-2502-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-017-2502-2