Abstract
Objective
To find out correlation between serum anti-tissue transglutaminase immunoglobulin-A (tTGA) levels and Marsh grading on duodenal histopathology in Celiac disease (CD).
Methods
In a prospective cohort study, a total of 52 symptomatic patients between age group of 2–18 y were enroled. All enroled patients were subjected to upper GI endoscopy by an experienced endoscopist. Two biopsies each from the bulb (D1) and second part (D2) of the duodenum were taken and Marsh grading was performed by a single experienced pathologist. Serum tTGA levels were also performed to find out correlation between serum tTGA levels and Marsh grading.
Results
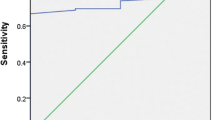
The mean age of the patients was 8.21 ± 3.45 y (Range: 2–16 y). Anemia was the most common non-gastrointestinal (GI) sign and was present in 73% of the cases. However the authors could not find out any significant association between Marsh grading and hemoglobin levels (r = 0.32, p > 0.05). Serum tTGA levels were found to be positively correlated with Marsh grading (Spearmen correlation coefficient ρ = 0.74, p 0.000). Significant differences were found in tTGA levels between different Marsh gradings (ANOVA test) (p 0.000). Receiver-operator curve (ROC) analysis cut-off value of serum tTGA for predicting villous atrophy was 178.8 (nine times of cut-off value) with sensitivity of 100% and specificity of 85.7%.
Conclusions
Serum tTGA levels can be used to predict villous atrophy and biopsy may be avoided in strongly suspected cases with more than 9 times of cut-offs.



Similar content being viewed by others
References
Ludvigsson JF, Leffler DA, Bai JC, et al. The Oslo definitions for celiac disease-related terms. Gut. 2013;62:43–52.
Sapone A, Bai JC, Ciacci C, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13–25.
Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–92.
Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology. 2001;120:636–51.
Ciclitira PJ. Celiac disease: a technical review. Gastroenterology. 2001;120:1526–40.
Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–43.
Catassi C, Fasano A. Celiac disease diagnosis: simple rules are better than complicated algorithms. Am J Med. 2010;123:691–3.
Lindfors K, Koskinen O, Kaukinen K. An update on the diagnostics of celiac disease. Int Rev Immunol. 2011;30:185–96.
Mubarak A, Nikkels P, Houwen R, Ten Kate F. Reproducibility of the histological diagnosis of celiac disease. Scand J Gastroenterol. 2011;46:1065–73.
Ravelli A, Villanacci V, Monfredini C, Martinazzi S, Grassi V, Manenti S. How patchy is patchy villous atrophy? Distribution pattern of histological lesions in the duodenum of children with celiac disease. Am J Gastroenterol. 2010;105:2103–10.
Pais WP, Duerksen DR, Pettigrew NM, Bernstein CN. How many duodenal biopsy specimens are required to make a diagnosis of celiac disease? Gastrointest Endosc. 2008;67:1082–7.
Husby S, Koletzko S, Korponay-Szabó IR, et al; ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastroenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–60.
Hill PG, Holmes GK. Coeliac disease: a biopsy is not always necessary for diagnosis. Aliment Pharmacol Ther. 2008;27:572–7.
Dahlbom I, Korponay-Szabó IR, Kovács JB, Szalai Z, Mäki M, Hansson T. Prediction of clinical and mucosal severity of coeliac disease and dermatitis herpetiformis by quantification of IgA/IgG serum antibodies to tissue transglutaminase. J Pediatr Gastroenterol Nutr. 2010;50:140–6.
Makharia GK, Verma AK, Amarchand R, et al. Prevalence of celiac disease in the northern part of India: a community based study. J Gastroenterol Hepatol. 2011;26:894–900.
Aro P, Ronkainen J, Storskrubb T, et al. Valid symptom reporting at upper endoscopy in a random sample of the Swedish adult general population: the Kalixanda study. Scand J Gastroenterol. 2004;39:1280–8.
Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney system. International workshop on the histopathology of gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–81.
Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–94.
Mubarak A, Wolters VM, Gmelig-Meyling FH, Ten Kate FJ, Houwen RH. Tissue transglutaminase levels above 100 U/mL and celiac disease: a prospective study. World J Gastroenterol. 2012;18:4399–403.
Fernández-Bañares F, Alsina M, Modolell I, et al. Are positive serum-IgA-tissue-transglutaminase antibodies enough to diagnose coeliac disease without a small bowel biopsy? Post-test probability of celiac disease. J Crohns Colitis. 2012;6:861–6.
Onyeador N, Jennings N, Paul SP, Ramani P, Sandhu BK. The relationship between tissue transglutaminase antibody titers and histological classification in celiac disease. Arch Dis Child. 2014;99:A34.
Vivas S, Ruiz de Morales JG, Riestra S, et al. Duodenal biopsy may be avoided when high transglutaminase antibody titers are present. World J Gastroenterol. 2009;15:4775–80.
Barker CC, Mitton C, Jevon G, Mock T. Can tissue transglutaminase antibody titers replace small-bowel biopsy to diagnose celiac disease in select pediatric populations? Pediatrics. 2005;115:1341–6.
Donaldson MR, Firth SD, Wimpee H, et al. Correlation of duodenal histology with tissue transglutaminase and endomysial antibody levels in pediatric celiac disease. Clin Gastroenterol Hepatol. 2007;5:567–73.
Mubarak A, Wolters VM, Gerritsen SA, Gmelig-Meyling FH, Ten Kate FJ, Houwen RH. A biopsy is not always necessary to diagnose celiac disease. J Pediatr Gastroenterol Nutr. 2011;52:554–7.
Trovato CM, Montuori M, Anania C, et al. Are ESPGHAN “biopsy sparing” guidelines for celiac disease also suitable for asymptomatic patients? Am J Gastroenterol. 2015;110:1485–9.
Contributions
RJ, VP and PS: Conceptualized the study, developed search strategy; VR: Did the study, analyzed the data and wrote the manuscript; SKV: Helped in preparing the manuscript. All authors approved the final version of manuscript. RJ will act as guarantor for the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Source of Funding
None.
Rights and permissions
About this article
Cite this article
Jora, R., Raghuvanshi, V., Payal, V. et al. Correlation of Tissue Transglutaminase with Modified Marsh Grading in Celiac Disease: A Prospective Cohort Study. Indian J Pediatr 84, 515–520 (2017). https://doi.org/10.1007/s12098-017-2323-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-017-2323-3